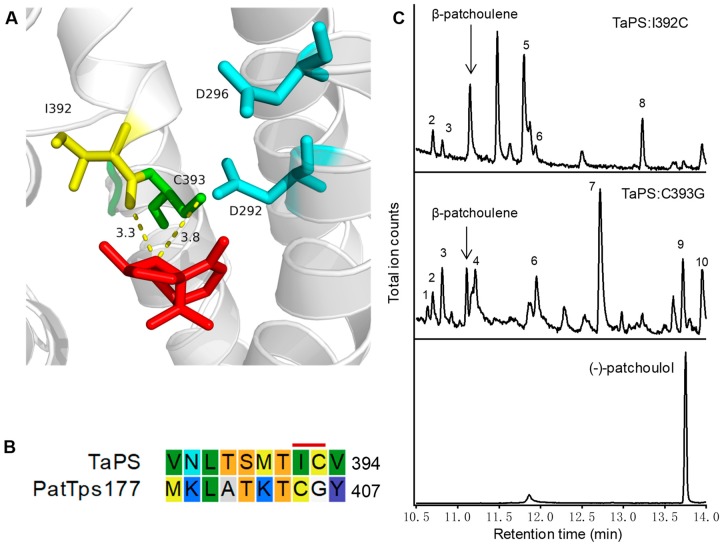
Figure 5.
Homology modeling of TaPS and site-directed mutagenesis analysis. (A) Catalytic cavity of TaPS was predicted based on homology modeling with docking of the final product β-patchoulene (red). Two key aspartates in the conserved DDXXD motif of terpene synthases were shown in blue. The two unique residues in TaPS, I392 and C393 were shown in yellow and green, respectively. The predicted distances between I392 or C393 and C2 of β-patchoulene were labeled as well (3.3 Å and 3.8 Å, respectively); (B) amino acid alignment of TaPS and patchoulol synthase (PatTPS177). The unique I392 and C393 were indicated by the red line. The full alignment was shown in Figure S4; (C) GC-MS chromatograms of TaPS:I392C and C393G with FPP as the substrate. The new products were labeled as peak 1–10 and their mass spectra were listed in Figure S5. The authentic (−)-patchoulol was injected for comparison.