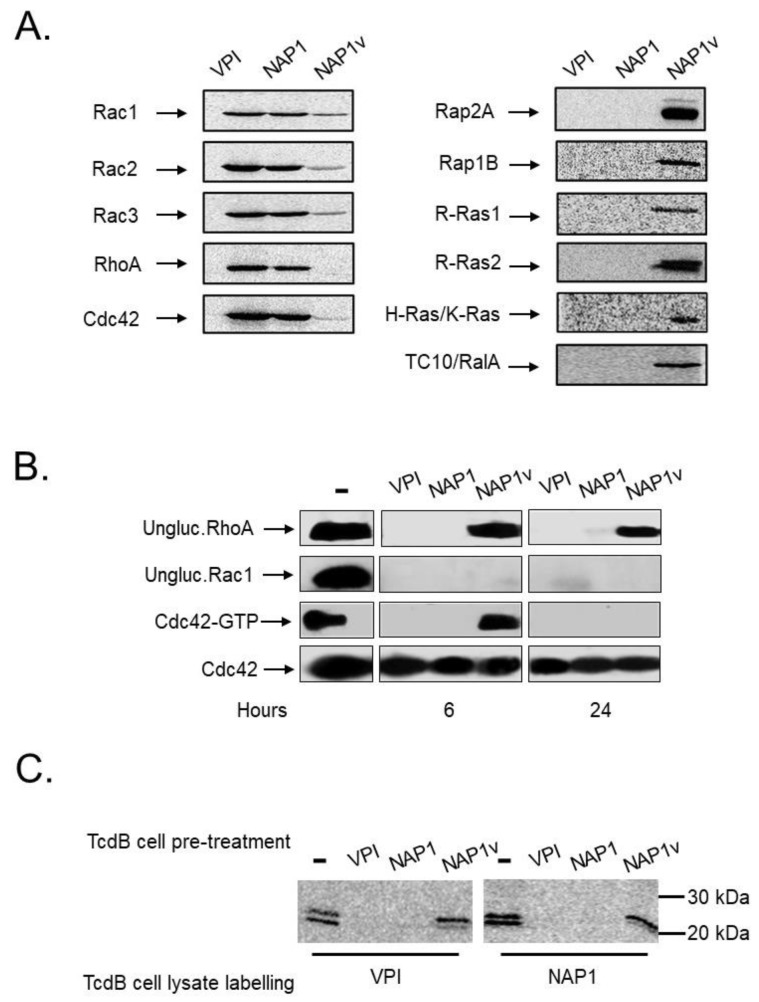
Figure 5.
Glucosyltransferase activity of TcdBs. (A) The in vitro glucosylation of distinct recombinant GTPases by TcdBVPI (VPI), TcdBNAP1 (NAP1), and TcdBNAP1v (NAP1v) was determined using UDP-[14C] glucose as a co-substrate. The reactions were incubated for 1 h and then subjected to SDS-PAGE. The radiolabeled bands were detected by phosphor imaging; (B) Following the addition of 100 pM of TcdB, HeLa cells were lysed at the indicated times, and the glucosylation of Rac1 and RhoA was monitored by immunoblot with specific anti-Rac1 and anti RhoA antibodies that only recognize the unmodified form of the proteins (ungluc.Rac1 and ungluc.RhoA, respectively). The effect of TcdBs on the activation state of Cdc42 was also evaluated. After intoxication, one part of the lysate was used as a control for the total amount of GTPase, and the rest was incubated with Rho Binding Domain–GST-sepharose beads. Cdc42 was detected by immunoblot with anti-Cdc42 antibodies; (C) HeLa cells were treated with 10 pM of TcdBVPI (VPI), TcdBNAP1 (NAP1), and TcdBNAP1v (NAP1v) for 12 h (TcdB cell pre-treatment). The cells were lysed, and the lysate proteins were glucosylated in vitro by TcdBVPI (VPI) or TcdBNAP1 (NAP1), according to the conditions stated in (A) (TcdB cell lysate labelling). Untreated cells (-) in (B) and (C) were included as a positive control for unmodified or activated proteins.