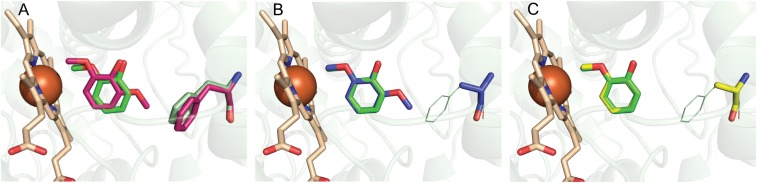
Fig. 2.
Structure-guided active site engineering of GcoA. Superpositions of WT and GcoA-F169A ligand-bound structures of GcoA, the P450 monooxygenase component of GcoAB, are shown. The heme is colored in bronze stick. (A) The guaiacol (green) and syringol (pink) complexes with WT GcoA are shown with the position of the GcoA-F169 residue highlighted. The translation and rotation of syringol compared with guaiacol result in a shift of the target methoxy carbon away from the heme. The Fe(III) to guaiacol methoxy carbon distance is 3.9 Å, and the Fe(III) to proximal syringol methoxy carbon distance is 4.3 Å. Data from PDB ID: 5NCB (28) and 5OMU (28). (B) The engineered GcoA-F169A-syringol complex (blue) enables positioning of the reactive methoxy group relative to the heme in a mode consistent with productive guaiacol binding. GcoA-F169 from the guaiacol-bound WT structure is shown in green lines. (C) Superposition of the WT (green) and GcoA-F169A (yellow) guaiacol-bound complexes reveals that guaiacol sits in an identical position in both crystal structures. GcoA-F169 from the guaiacol-bound WT structure is shown in green lines.