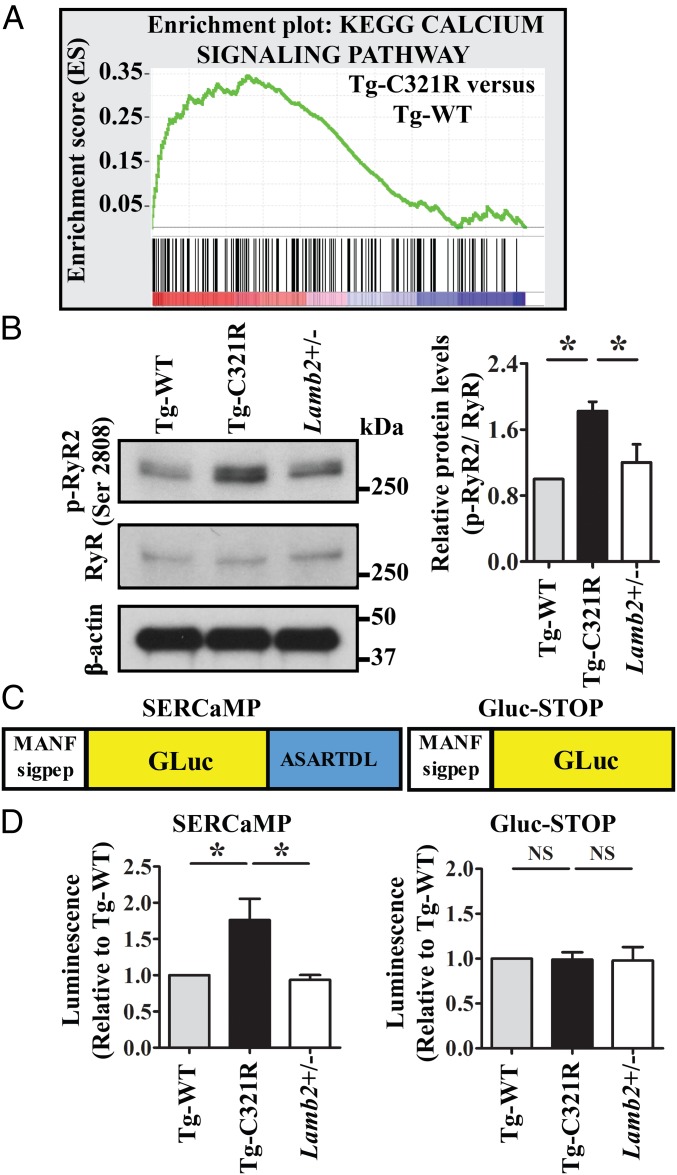
Fig. 4.
Podocyte ER stress induces ER calcium release through RyR2 phosphorylation. (A) GSEA of calcium-signaling gene transcript abundance in primary podocytes (P0) isolated from Tg-C321R and Tg-WT mice at P27 (enrichment score = 0.34, normalized enrichment score = 1.41). P < 0.001. (B) WB analysis of phospho-RyR2 (p-RyR2; Ser2808) and RyR expression in primary podocyte lysates from the indicated genotypes. Quantification of p-RyR2 (Ser2808) was normalized to RyR in the podocyte lysates. Mean ± SD of 3 independent experiments. *P < 0.05 by ANOVA. (C) Schematic of SERCaMP and control GLuc-STOP fusion proteins. The first 18 amino acids of GLuc (MGVKVLFALICIAVAEAK, signal peptide) were replaced with the signal peptide of MANF (amino acids 1–23, MWATQGLAVALALSVLPGSRALR) to target the proteins to the secretory pathway. The C-terminal sequence of MANF, ASARTDL, was fused to GLuc. (D) Primary podocytes from Tg-WT, Tg-C321R, and Lamb2+/− mice at P27 were transduced with lentivirus expressing SERCaMP or GLuc-STOP. Forty-eight h after the virus infection, luciferase activity was measured in the medium and normalized to the value in Tg-WT mice. Mean ± SD from 3 independent experiments. *P < 0.05. NS, not significant by ANOVA.