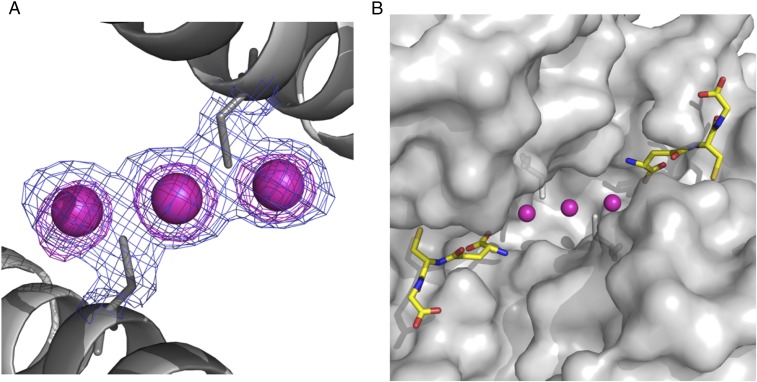
Fig. 4.
cis-DDP can be sequestered by GST P1-1, in the presence of GSH, by binding at the dimer interface of the enzyme. (A) Final (2Fo − Fc) electron density map (contour level 1σ in blue) and anomalous difference Fourier maps (contour at 4σ in pink) focused on the dimer interface. The Pt ions are designated by the purple spheres. (B) Surface representation showing the Pt-binding site in relation to the active sites. The purple spheres are the Pt ions, and GSH is shown in stick fashion with carbon bonds in yellow, nitrogen atoms in dark blue, oxygen atoms in red, and sulfur atoms in gold.