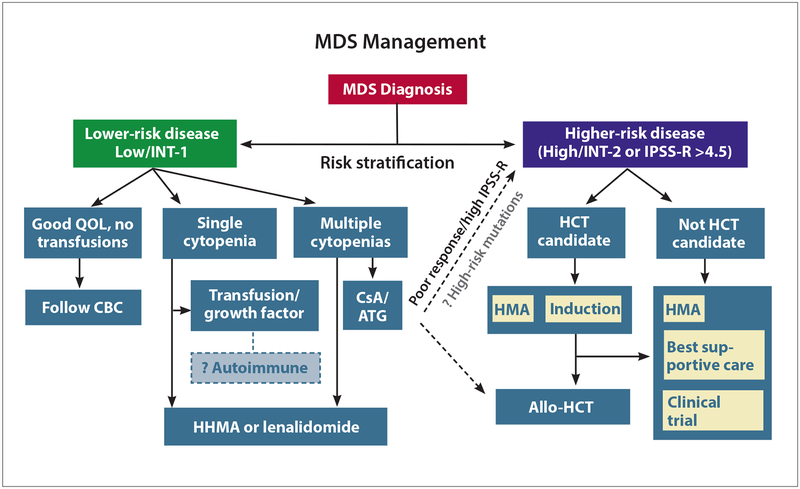
Figure.
Approach to therapeutics in myelodysplastic syndromes. Clinical trial enrollment should be considered at all stages. Patients should be risk stratified with a tool such as the IPSS-R. Some patients with lower-risk disease who have mild cytopenias and are minimally symptomatic may just be observed with serial CBCs. Patients who have anemia alone can be administered an erythropoiesis-stimulating agent if their serum erythropoietin level is less than 500 U/L, or can receive lenalidomide if they have del(5q). The approach for other patients is challenging and may involve immunosuppressive therapy with CsA and ATG, a combination growth factor approach, lenalidomide despite the absence of del(5q), or a hypomethylating agent. For patients with higher-risk disease, the key decision is whether the patient is an allogeneic stem cell transplant candidate. If the patient is a transplant candidate, the transplant should be performed as soon as possible, potentially with a hypomethylating agent as a bridge. If not, the patient should receive therapy with a hypomethylating agent; in this setting, azacitidine has improved survival compared with conventional care. “Induction” might include CPX-351 instead of the conventional cytarabine-anthracycline “3&7” combination.
Allo-HCT, allogeneic hematopoietic stem cell transplant; ATG, antithymocyte globulin; CBC, complete blood cell count; CsA, cyclosporine-A; HCT, hematopoietic stem cell transplant; HMA, hypomethylating agent; INT, intermediate risk; IPSS, International Prognostic Scoring System; IPSS-R, Revised International Prognostic Scoring System; MDS, myelodysplastic syndromes; QOL, quality of life.