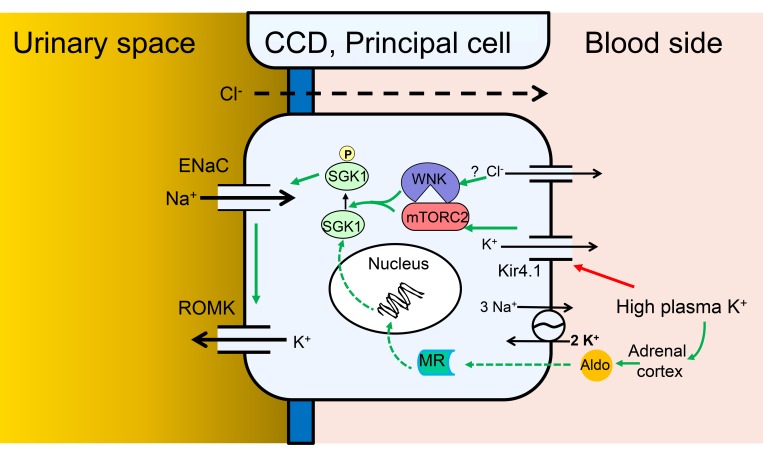
Figure 9. Model of Na+ and K+ transport regulation by local and systemic [K+] in PCs of the CCD.
Cellular model of the integrated regulation of ENaC activity, and ENaC-dependent K+ secretion, in response to altered plasma K+. Green arrows indicate stimulatory effects. Red arrow indicates inhibitory effect. Solid arrows indicate rapid direct effects (seconds to minutes), dashed arrows indicate slower genomic effects (minutes to hours). According to this scheme, elevation of basolateral [K+] triggers membrane depolarization by altering the K+ channel–dominated resting potential, which with a possible involvement of intracellular [Cl–] and WNK1 stimulates mTORC2-dependent SGK1 activation via HM phosphorylation. Activated SGK1 stimulates ENaC predominately by inhibiting Nedd4-2, but also potentially through other effects including ENaC phosphorylation. The ensuing increase in electrogenic Na+ transport enhances K+ secretion via K+ channels, predominantly renal outer medullary K+ (ROMK) channels, but also large-conductance Ca2-activated K+ (BK) channels. According to the model, SGK1 acts as a signal integrator, responding both to local signals that increase its activity, and to systemic hormonal signals (primarily aldosterone), which increase its expression.