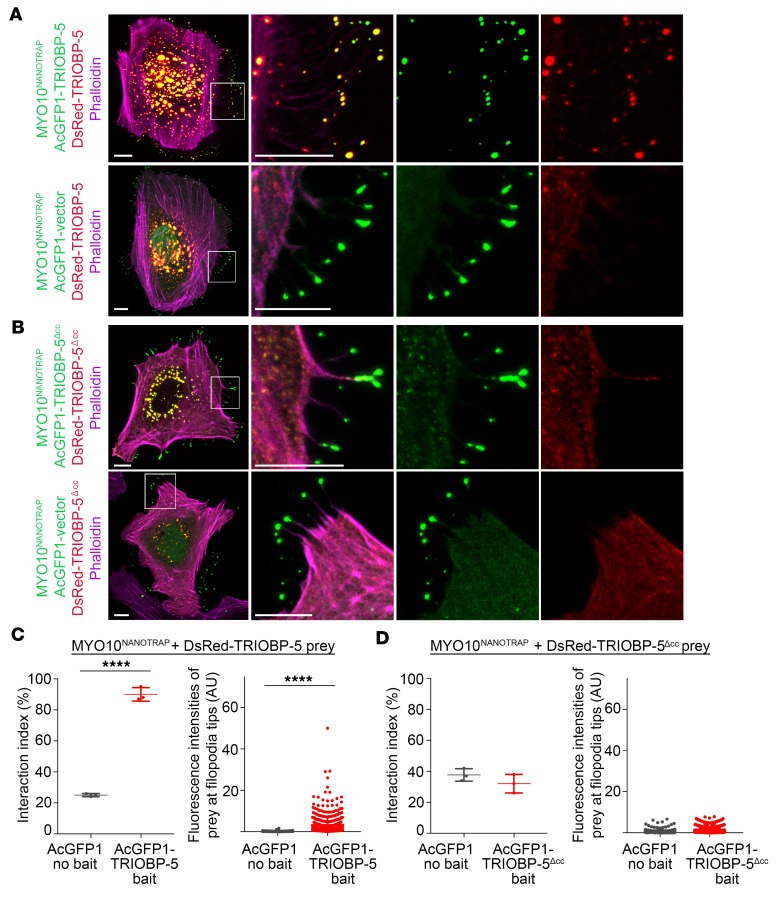
Figure 7. TRIOBP-5 homodimerization revealed by nanoscale pull-down assays.
(A) MYO10-HMM-Nanotrap (abbreviated MYO10NANOTRAP) specifically binds GFP derivatives, but not DsRed, and traffics GFP-tagged protein to filopodia tips. Representative image of a HeLa cell transfected with 3 expression vectors encoding MYO10NANOTRAP, AcGFP1-TRIOBP-5, and DsRed-TRIOBP-5 (upper panel), and a control HeLa cell transfected with the MYO10NANOTRAP, AcGFP1 vector, and DsRed-TRIOBP-5 (lower panel). AcGFP1-TRIOBP-5 and DsRed-TRIOBP-5 colocalize to filopodia tips (upper panel), while in the control, only the AcGFP1 vector localized at filopodia tips (lower panel). The complex of MYO10NANOTRAP and AcGFP1-TRIOBP-5 partnered with and transported DsRed-TRIOBP-5 to filopodia tips. The complex of MYO10NANOTRAP and AcGFP1 vector did not traffic DsRed-TRIOBP-5. These data indicate that TRIOBP-5 can homomultimerize in vivo. The second column shows enlarged images of the white-outlined areas from the first column. The third and fourth columns are the individual green and red channels of merged images in the second column. (B) Transfected HeLa cell with MYO10NANOTRAP, AcGFP1-TRIOBP-5Δcc, and DsRed-TRIOBP-5Δcc (upper panel), and control HeLa cell transfected with MYO10NANOTRAP, AcGFP1 vector, and DsRed-TRIOBP-5Δcc (lower panel). Triobp-5Δcc is deleted for sequence encoding the coiled-coil domains and 78 bp of upstream sequence. DsRed-TRIOBP-5Δcc was not trafficked to filopodia tips by the MYO10NANOTRAP and AcGFP1-TRIOBP-5Δcc, indicating that TRIOBP-5Δcc does not multimerize. (C) Left panel: Quantification of AcGFP1-TRIOBP-5 or AcGFP1 vector binding to DsRed-TRIOBP-5. When MYO10NANOTRAP, AcGFP1-TRIOBP-5, and DsRed-TRIOBP-5 were coexpressed, 90% ± 4.4% of filopodia (3 independent experiments) showed a statistically significant correlation between green and red fluorescence at filopodia tips (2-tailed t test). Right panel: Fluorescence intensities of DsRed-TRIOBP-5 at filopodia tips. The presence of AcGFP1-TRIOBP-5 caused a significant increase of fluorescence intensity of DsRed-TRIOBP-5 at the filopodia tips (Mann-Whitney U test). (D) Interaction index of either AcGFP1-TRIOBP-5Δcc or AcGFP1 vector with DsRed-TRIOBP-5Δcc (left panel). AcGFP1-TRIOBP-5Δcc does not bind to DsRed-TRIOBP-5Δcc. Fluorescence intensities of DsRed-TRIOBP-5Δcc at filopodia tips (right panel). AcGFP1-TRIOBP-5Δcc does not increase the intensity of DsRed-TRIOBP-5Δcc at filopodia tips, indistinguishable from the AcGFP1 vector control experiment. Scale bars: 10 μm. Data are mean ± SD. ****P < 0.0001.