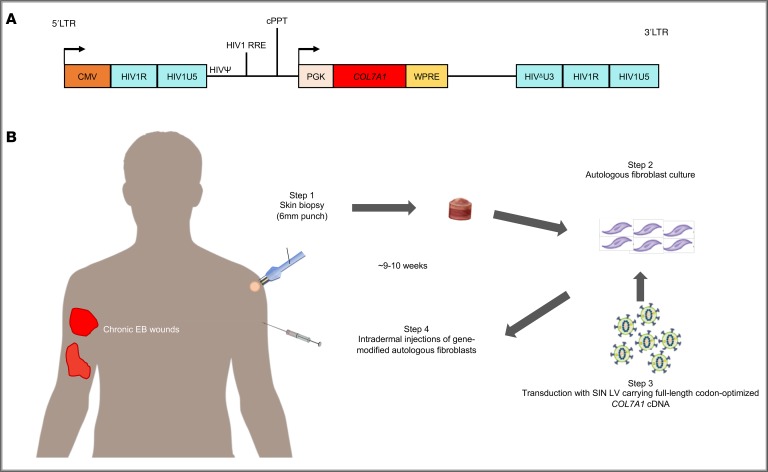
Figure 1. Illustration of Good Manufacturing Practice–compliant production of COL7A1-supplemented autologous fibroblasts for patients with recessive dystrophic epidermolysis bullosa in the present study (also referred to as the LENTICOL-F Trial).
(A) Configuration of a third-generation, 4-plasmid-system, self-inactivating lentiviral vector with deleted U3 region of the 3′ LTR, carrying full-length codon-optimized COL7A1 cDNA (SIN LV–coCOL7A1). An internal PGK promoter, mutated Woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), and HIV central polypurine tract (cPPT) are shown. (B) Steps involved in the GMP manufacture of ex vivo gene-modified autologous fibroblasts for intradermal injections in a subject with recessive dystrophic epidermolysis bullosa (RDEB). It took approximately 9–10 weeks from the time of obtaining the skin biopsy sample to the time of treatment.