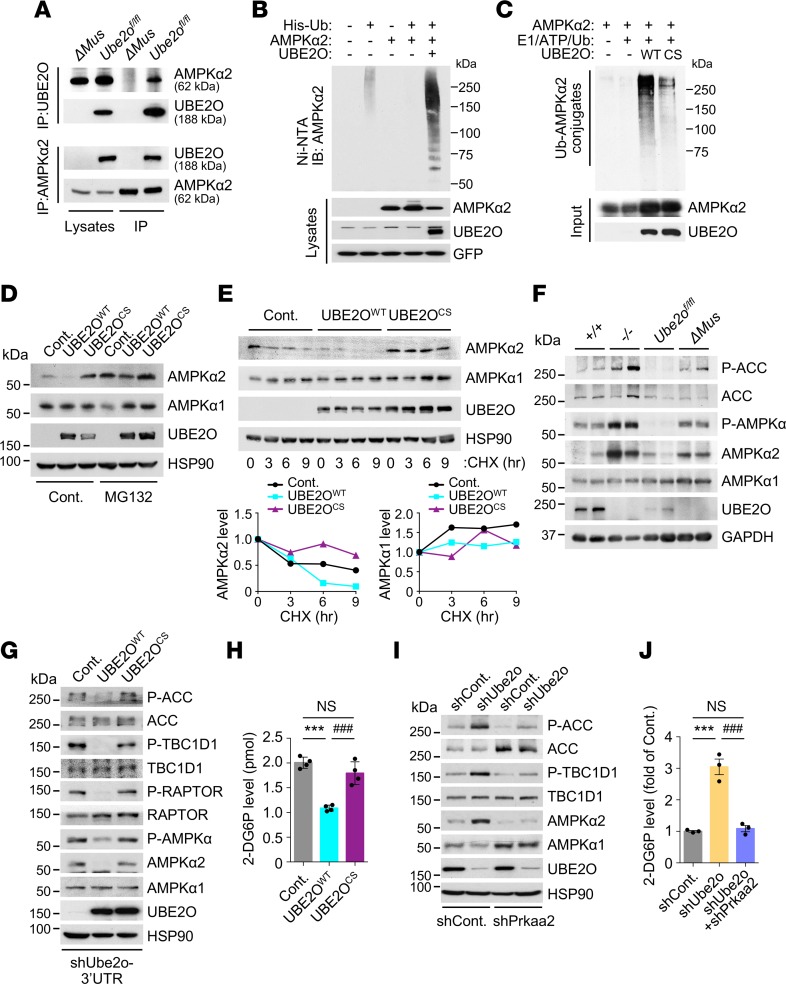
Figure 6. Regulation of AMPKα2 by skeletal muscle UBE2O.
(A) UBE2O (top) or AMPKα2 (bottom) immunoprecipitates of lysates from skeletal muscle of control (Ube2ofl/fl) and Ube2oΔMus mice were subjected to immunoblotting for AMPKα2 or UBE2O. (B) Lysates from C2C12 myotubes transfected with the indicated plasmids were subjected to metal affinity purification for His-tagged ubiquitin (His-Ub), then immunoblotting for ubiquitinated AMPKα2. Ni-NTA, Ni2+-nitrilotriacetic acid. (C) Recombinant AMPKα2 proteins were subjected to in vitro ubiquitination assay in the presence of in vitro translated WT or C1037S (CS) mutant UBE2O. (D) Lysates from C2C12 myotubes expressing WT or CS mutant UBE2O treated with MG132 (10 μM) for 6 hours were subjected to immunoblotting. (E) Lysates from C2C12 myotubes expressing WT or CS mutant UBE2O treated with cycloheximide (CHX) for the indicated times were subjected to immunoblotting (top). AMPKα2 or AMPKα1 protein levels were quantified by normalizing to the intensity of the HSP90 band (bottom). (F) Lysates from skeletal muscle of 12-week-old Ube2o+/+, Ube2o–/–, Ube2ofl/fl, and Ube2oΔMus mice were subjected to immunoblotting for the indicated proteins. (G) Lysates from C2C12 myotubes expressing UBE2O shRNA together with exogenous WT or CS mutant UBE2O from a Ube2o ORF transcript lacking the 3′ UTR sequence targeted by shRNA (shUbe2o-3′UTR) were subjected to immunoblotting for the indicated proteins. (H) Insulin-stimulated glucose uptake rate of C2C12 myotubes from G. 2-DG6P, 2-deoxyglucose-6-phosphate. n = 4. (I) Lysates from C2C12 myotubes expressing Ube2o shRNA together with Prkaa2 shRNA were subjected to immunoblotting for the indicated proteins. (J) Insulin-stimulated glucose uptake rate of C2C12 myotubes from I. n = 3. Error bars represent ±SEM. P value was determined by ANOVA (***P < 0.001, ###P < 0.001).