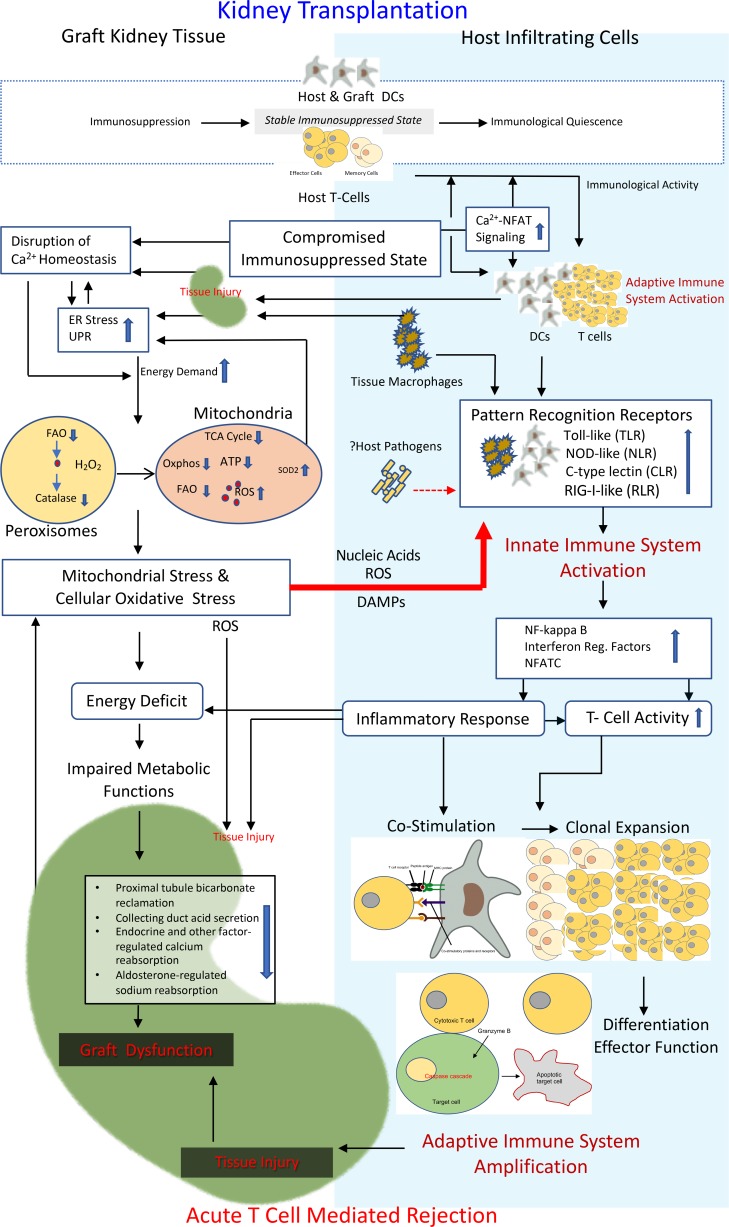
Figure 11. A model for innate immune system activation in TCMR.
At transplantation, allograft resident donor DCs migrate to host secondary lymphoid organs and function as antigen-transporting cells. Host T cells recognize intact donor HLA on donor DCs (direct allorecognition) or host DCs (semidirect allorecognition). Donor HLA can also be internalized by host DCs, processed, and presented as peptide fragments along with host HLA; self-MHC-restricted host T cells then recognize the peptide fragments presented by the host DCs (indirect allorecognition). Before antigen presentation, DCs, the key component of innate immune system, need to transform from an immature antigen-capture phenotype to a mature T cell–activation phenotype. Peritransplant tissue injury and ensuing inflammatory microenvironment cause DC maturation and priming of the adaptive immune system, resulting in clonal expansion and differentiation of T cells. Immunosuppressive drug therapy keeps the immune system relatively quiescent. Any compromise to this stable immunosuppressed state (Figure 8) activates alloreactive T cells that cause tissue injury, probably at a low level. In this context, increased cellular calcium flux (Supplemental Tables 11 and 12) results in increased ER stress (Supplemental Table 13). The high energy demand leads to increased cellular oxidative stress (Supplemental Table 14) and mitochondrial stress, resulting in reduced cellular peroxisomes and mitochondrial energy production (Supplemental Tables 15 and 16), and generation of reactive oxygen species. Products of cellular damage (DAMPs; Supplemental Tables 8–10) are sensed by PRRs (Supplemental Tables 2–7), predominantly expressed on DCs and macrophages, leading to a full-fledged activation of the innate immune system. The resultant inflammatory response generates costimulatory signals (Figure 10) for T cell clonal expansion and differentiation. The energy deficit compromises several key metabolic pathways within the graft (Figure 9), resulting in graft dysfunction. Tissue injury and graft dysfunction further increases the cellular stress, thus amplifying the feed-forward loop.