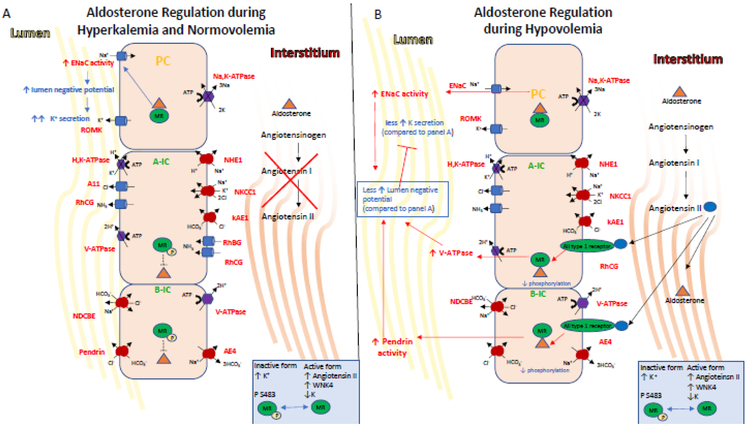
Figure 3. Endocrine regulation of IC transport.
The mineralocorticoid receptor is one of the end targets of the RAAS. These hormones elicit signaling events in the kidney that regulate K+ and salt homeostasis, intravascular volume and cell metabolism.
A) Indirectly during hyperkalemia, aldosterone is secreted and its stimulation of ENaC via MR leads to lumen electronegativity, which promotes K+ secretion via ROMK expressed in PCs and the BK channel (not shown here), which is expressed in ICs. Aldosterone also indirectly increases acid excretion by the kidney as the net lumen-negative transepithelial voltage makes secretion of H+ by the ICs more favorable. Aldosterone, facilitated by the MR receptor, also regulates activity in ICs. Aldosterone action on the MR receptor in ICs is modulated by phosphorylation at Ser-483, a modification that reduces affinity and activation of the receptor for aldosterone. Importantly, hyperkalemia also induces MR phosphorylation at Ser-483, thus inhibiting aldosterone-stimulated V-ATPase in A-ICs and the pendrin in B-ICs. This diminished Cl− reabsorption limits net NaCl reabsorption, and thus allows for aldosterone to promote K+ secretion while limiting volume expansion.
B) On the other hand, Angiotensin II, WNK4 activity and hypokalemia induce decreased levels of phosphorylated MR, allowing aldosterone binding to MR which increases the coordinated action of the V-ATPase and pendrin. This coordinated action increases plasma volume while inhibiting K+ secretion via increase of electroneutral NaCl reabsorption and limiting the lumennegative potential, the driving force for K+ secretion.