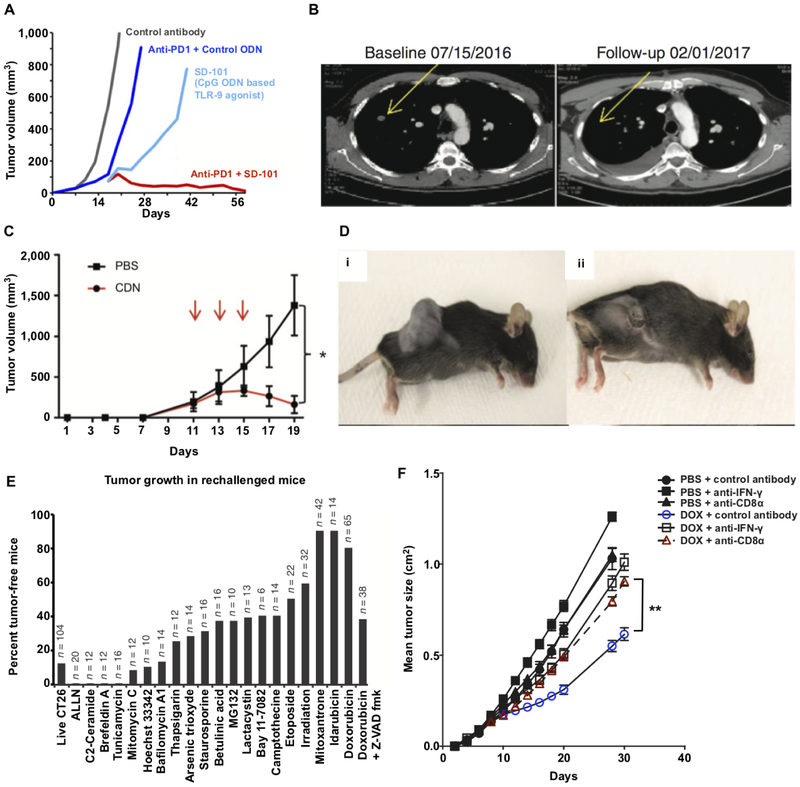
Figure 2: In situ vaccination with immunostimulants and chemotherapeutics can promote durable tumor regression through immunological mechanisms.
(A) Mice with established CT26 tumors received either intratumoral (i.t.) injections with a CpG oligodeoxynucleotide (ODN)-based TLR-9 agonist, SD-101, i.t. SD-101 + systemic anti-PD1, i.t. control (inactive) ODN + systemic anti-PD1 or i.t. control ODN alone. Tumor growth kinetics are shown and indicate that i.t. treatment with the TLR-9 agonist (SD-101) combined with anti-PD1 leads to optimal tumor regression. (B) CT images taken from a patient with metastatic melanoma that was treated with SD-101 and anti-PD1 show regression of a non-injected tumor, suggesting that the treatment induced systemic anti-cancer T cell immunity. (C, D) Intratumoral injection of a CDN-based STING agonist leads to acute rejection of B16F10 melanoma tumors. Mice were implanted with 5×105 B16F10 cells on day 0 and then treated with either CDN or PBS for a total of three treatments (red arrows). Tumor growth kinetic (C) and representative images (D) of mice treated with PBS (i) or CDN (ii) are shown. (E) CT26 tumor cells were cultured with the indicated chemotherapeutic agents (x-axis) for 24-48 hours and then injected into the left flank of mice. Live tumor cells were injected into the right flank of the same mice 8 days later and the percentage of tumor-free mice was assessed 120 days later (note: n is the total number of mice used across multiple studies). Tumor rejection indicates that anti-cancer T cell immunity was primed by the treated cells, which suggests that the treatment promoted immunogenic cell death. (F) Mice with established MCA205 fibrosarcoma cells were treated with intratumoral doxorubicin or PBS and a subset of these mice were treated with either anti-IFN-γ or anti-CD8 antibodies to evaluate the impact of IFN-γ and CD8 T cell depletion on tumor regression by doxorubicin. Panel (A) adapted from ref. [34]; (B) adapted from ref. [37]; (C,D) adapted from ref. [41]; (E) adapted from ref. 48; and, (F) adapted from ref. 47.