Abstract
Prenatal alcohol exposure is the leading cause of birth defects, collectively termed fetal alcohol spectrum disorders (FASD). In the United States and Canada, 1 in 100 children will be born with FASD. Some of the most commonly debilitating defects of FASD are in social behavior. Zebrafish are highly social animals, and embryonic ethanol exposure from 24 to 26 hours post‐fertilization disrupts this social (shoaling) response in adult zebrafish. Recent findings have suggested that social behaviors are present in zebrafish larvae as young as 3 weeks, but how they relate to adult shoaling is unclear. We tested the same ethanol‐exposed zebrafish for social impairments at 3 weeks then again at 16 weeks. At both ages, live conspecifics were used to elicit a social response. We did not find alcohol‐induced differences in behavior in 3‐week‐old fish when they were able to see conspecifics. We do find evidence that control zebrafish are able to use nonvisual stimuli to detect conspecifics, and this behavior is disrupted in the alcohol‐exposed fish. As adults, these fish displayed a significant decrease in social behavior when conspecifics are visible. This surprising finding demonstrates that the adult and larval social behaviors are, at least partly, separable. Future work will investigate the nature of these nonvisual cues and how the neurocircuitry differs between the larval and adult social behaviors.
Keywords: adult, fetal alcohol spectrum disorder, larva, social behavior, zebrafish
Introduction
Across the world harmful alcohol (ethanol, ethyl alcohol) use is one of the top five contributors to disability, disease and death (World Health Organization 2014). Furthermore, prenatal alcohol exposure can leave a developing fetus with different life‐long disorders of varying severity (Abel 1996). The term fetal alcohol spectrum disorder (FASD) is used to describe all the disorders caused by prenatal alcohol exposure (Stratton, Howe & Battaglia 1996).
Fetal alcohol spectrum disorder is a widespread and devastating issue. For example, in the United States, autism spectrum disorder has an estimated prevalence ranging between 1.4% and 1.5% (Christensen et al. 2016). In comparison, FASD in the United States, Italy and South Africa FASD has a prevalence rate between 2% and 5% (May et al. 2014), which still may be an underestimate. In the United States, for example, 45% of pregnancies are unintended (Finer & Zolna 2016) and 51.5% of women of childbearing age (18 to 44) report consuming at least one alcoholic drink in the previous 30 days (Centers for Disease Control and Prevention 2012). Collectively, this suggests that, in the United States and likely elsewhere, there are a large number of prenatal alcohol exposures.
Prenatal alcohol exposure severely decreases the quality of life for individuals with FASD. Individuals with FASD often display impairments in behavioral and cognitive function, such as impaired learning, attention and memory or socialability. Individuals with FASD have trouble with perceiving social cues (Kodituwakku 2007; Rasmussen et al. 2011). They also lack the suitable initiative to form and/or maintain reciprocal friendships (Roebuck, Mattson & Riley 1999). Not only do such social deficits directly hamper the ability of individuals with FASD to form social relations or obtain employment but can also lead to trouble with the law, inappropriate sexual relationships, suicide and depression (Kelly, Day & Streissguth 2000; Kully‐Martens et al. 2012). Thus, it is critical that we understand the causes of FASD and how prenatal alcohol exposure disrupts the genesis of social behaviors.
The zebrafish has emerged as model organism in which multiple aspects of FASD can be examined (Fernandes, Buckley & Eberhart 2017). For example, embryonic alcohol exposure causes growth abnormalities and craniofacial defects in zebrafish (Bilotta et al. 2004; Carvan et al. 2004; McCarthy et al. 2013). Furthermore, zebrafish are highly social, making them potentially useful for understanding how ethanol exposure alters this behavior. Embryonic alcohol exposure alters social behavior in zebrafish across strains (AB and TU) and likely throughout adulthood, at least from 4 months (Parker et al. 2016) up to 24 months (Fernandes, Rampersad & Gerlai 2015b). However, we know little regarding the genesis of these behavioral defects. Recently, 3‐week‐old zebrafish larvae have been shown to exhibit social‐like behaviors (Dreosti et al. 2015). It remains unknown how related the larval and adult behaviors are and whether or not the larval behavior is disrupted by ethanol exposure.
The goal of the current work was to examine the effect of embryonic alcohol exposure on the social preference of the same zebrafish at two different ages: 3 and 16 weeks. Groups of live, age‐matched conspecifics were used to examine the social responses at larval and adult stages. Our results demonstrate that at 3 weeks of age, untreated, but not ethanol‐treated, embryos can use nonvisual stimuli to detect other zebrafish. When provided visual stimuli, ethanol‐treated and ethanol‐untreated zebrafish behaved identically, with a strong preference to be close to other zebrafish. However, at 16 weeks of age, these same fish exhibited a significantly less robust shoaling response to a visual stimulus of other zebrafish. Our results, along with those of others, suggest that adult social behavior is more severely disrupted by embryonic alcohol exposure than larval social behavior.
Materials and Methods
Zebrafish (Danio rerio) care and use
All embryos were raised and cared for using established Institutional Animal Care and Use Committee (IACUC) protocols (Westerfield 1993) approved by the University of Texas at Austin. Wild‐type AB strain of zebrafish was staged using a published staging guide for zebrafish (Kimmel et al. 1995). Our goal was to use a concentration that was shown to impair adult social behavior, to determine if the same concentration impaired larval social behavior. Previous research has demonstrated that a 1y% ethanol dose from 24 to 26 hours post‐fertilization impairs adult social behavior (Fernandes & Gerlai 2009; Buske & Gerlai 2011; Fernandes, Rampersad & Gerlai 2015a; Fernandes et al. 2015b). Additionally, we added a higher dose to determine whether ethanol had a floor or ceiling effect on social behavior. We treated chorinated embryos with 1% or 1.5% ethanol, approximately 41‐ and 62‐mM tissue levels (Lovely, Nobles & Eberhart 2014), in embryo medium from 24 to 26 hours post‐fertilization. Behavioral testing was first conducted at 3 weeks of age (larval stage), and the same fish were retested when they were 16‐week‐old (adult).
Behavioral apparatus
Larval arena
The social behavior of 3‐week‐old zebrafish was assayed using an in‐house designed custom built behavioral arena [Fig. 1(a)] fashioned on a design from Dreosti et al. (2015). The walls of the arena were built with 6‐mm white Plexiglas while 5.5‐mm transparent Plexiglas was used for the floor. Laser cut sections of the arena were bound together using epoxy. A single arena measured (6.5 × 3.3 × 1 cm, Length × Width × Height). The arena formed a U‐Shape. Each arm of the arena measured 2.1 × 3.3 × 1 cm. Each arm was divided into two sections by two dividers: a transparent divider followed by an opaque divider. The viewing chamber and the housing chamber each measured 2.1 × 1.3 × 1 cm (L × W × H). The housing chamber held two age matched fish that were used to elicit a social response from test subjects. During a trial, only one of the two housing chambers held fish, while the other contained only fish water. The width of the passage connecting the two arms of the arena was 0.6 cm. Six arenas were used at once and were placed on a transparent base. A 15‐W fluorescent light tube was placed directly below the arenas. White paper was placed on the bottom of the transparent base that held the six arenas, to increase contrast. A Basler acA1300‐60gc, video camera was placed directly above the arenas. Recorded trials which were analyzed in real time via Ethovision Video tracking software (Version 11.5 XT, Noldus Info Tech, Wageningen, the Netherlands).
Figure 1.
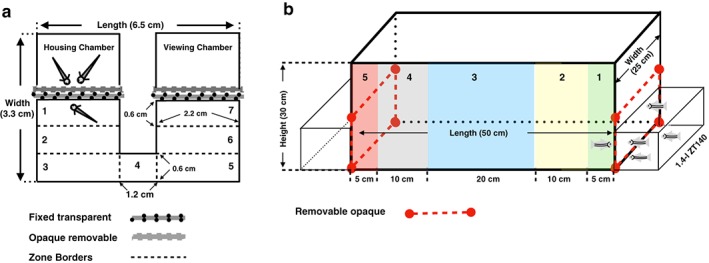
Schematic representations of larval and adult behavioral apparatus. (a) Apparatus used to assay social preference in 3‐week‐old zebrafish. All zones except zone 4 have identical dimensions. Zones were assigned by using Ethovision. (b) Apparatus used to assay social behavior in 16‐week‐old zebrafish. Each color marks a different zone, labeled 1 through 5
Adult arena
Adult social arenas consisted of a 37‐l tank (50 × 25 × 30 cm, L × W × H) with 1.4‐l ZT140 Aquaneering tanks placed outside along the width of the tank [Fig. 1(b)]. During the trial one of the two tanks, the stimulus side held four conspecific fish (2 males and 2 females) while the other tank only contained water. The outer wall of these tanks was coated with white corrugated plastic to be consistent with the experimental tank and to block any other potential cues. White opaque barriers were placed between the 1.4‐l tanks and 37‐l tank. The experimental tank was illuminated by a 15‐W fluorescent light tube placed directly above it. The back side and bottom of the 37‐l tank were coated with white corrugated plastic sheets to increase the contrast, reduce glare and reflections for video‐tracking analysis. Two identical arenas were used in parallel. The behaviors of the experimental fish were recorded and analyzed in real time using Ethovision.
Behavioral test procedure
Larval behavioral assay
To explore the potential effect of early developmental exposure to alcohol on larval social behavior, we placed 3‐week‐old zebrafish into the larval arena singly, and 30 seconds later, we started a 30‐minute long recording session. During the recording session, the test subject was first presented with a 15‐minute habituation period. During habituation, an opaque divider prevented the test subject from viewing the conspecifics that were in the housing chamber. After 15 minutes, the opaque divider was manually lifted, and test subjects were able to view the conspecifics that were present in one arm of the arena. The stimuli were presented only in one arm for each experimental fish but the arm of presentation alternated randomly across experimental subjects.
Adult social assay
We placed adult experimental zebrafish into the test tank singly, and 30 seconds later, we started a 20‐minute long recording session. During the recording session, the subject was first presented with a 10‐minute habituation period, followed by a 10‐minute period during which test subjects could visualize a shoal of zebrafish (2 males and 2 females) in an adjacent tank. As with the larval assays, the side of the tank nearest the shoal alternated randomly across experimental subjects.
Quantification of behavior and statistical analysis
Data were analyzed using SPSS (version 23) for the Mac.
Larval behavior
Video files were recorded and analyzed in real time with Ethovision XT. The arena was divided into seven zones for analysis [Fig. 1(a)]. To determine whether embryonic alcohol exposure affected locomotor activity, we compared the total distance moved during the entire 30‐minute trial between treatment groups. We used a one‐way analysis of variance (ANOVA) to determine whether alcohol treatment (between‐subject factor) affected the total distance moved. In these analyses, the assumptions of homogeneity of variance was violated [Levene's test F(2, 43) = 5.85, P = 0.006], Therefore, the Welch F statistics was reported. Subsequently, the Games‐Howell post hoc test was used to determine group differences.
We measured the time fish spent in each zone during habituation and stimulus presentation to determine whether embryonic alcohol exposure affected the preference for the live shoal. Separate mixed ANOVA were used to determine the effect zone (7, repeated measure factor), alcohol treatment (three conditions; between‐subject variable) and their interactions had on the percentage of time 3‐week‐old zebrafish spent near the live shoal during habituation and stimulus presentation. According to Mauchly's test, the assumption of sphericity was violated with the data from the habituation period [χ2(20) = 189.97, P < 0.001] and the stimulus period [χ2(20) = 337.21, P < 0.001]. Thus, the degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = 0.41 and ε = 0.27, respectively). Given the inappropriateness of post hoc multiple comparison tests such as Tukey Honestly Significant Difference (HSD) for the analysis of a repeated measure variables such as zone, we separately compared the time spent in zone 1 between treatment groups during habituation and stimulus presentation.
Adult behavior
We quantified social preference by measuring the distance the fish maintained from the live shoal adjacent to their experimental tank (average distance from stimulus in cm). A mixed‐model ANOVA was used to investigate the effect of interval (20, 1‐minute intervals, the repeated measure factor), alcohol treatment (three conditions; between‐subject variable) and their interactions had on the mean distance to the live shoal. The assumption of sphericity was violated in mean distance from stimulus [χ2(189) = 347.91, P < 0.001] so the degrees of freedom were corrected as above (ε = 0.42). Because multiple comparison post hoc tests such as Tukey's HSD are inappropriate for the analysis of a repeated measure, we calculated mean reduction in distance to stimulus. A one‐way ANOVA followed by Tukey's post hoc test was used to examine the effect embryonic alcohol exposure had on the average reduction in the distance to stimulus. Statistical analysis of adult fish behavior demonstrated no significant Sex differences [F(1, 29) = 0.002, P = 0.96], Sex × Interval interaction [F(7.95, 230.53) = 0.74, P = 0.78], Sex × Alcohol treatment interaction [F(2, 29) = 2.12, P = 0.14] or Sex × Interval × Alcohol treatment interaction [F(15.99, 230.53) = 1.28, P = 0.12]. As a result, data are pooled for sex in subsequent analyses.
To determine whether alcohol exposure impacted locomotor activity, we examined the time fish spent in various zones during habituation (live shoal not visible). A mixed ANOVA was used to investigate the effect of zone (5, repeated measure factor), alcohol treatment (three conditions; between‐subject variable) and their interactions had on the percentage of time spent across zones. Again, sphericity was violated [χ2(189) = 347.91, P < 0.001] and the degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity (ε = 0.42).
We also sought to determine whether alcohol impaired the response to the visual presentation of the stimulus. Therefore, we examined the amount of time it took a fish to reach the zone closest to the live shoal (latency) using a one‐way ANOVA.
Results
Three‐week‐old zebrafish (larval) behavior
We first sought to determine if embryonic ethanol exposure altered the amount larval fish swam, which could impact our behavioral analyses. Over the course of the 30‐minute trial, fish treated with 1.0% ethanol swam the least (M = 829.41, SD = 352.61), while fish treated with 1.5% ethanol swam slightly further (M = 1241.93, SD = 281.33) than controls (M = 1147.33, SD = 496.71) (Fig. 2). There was a significant effect of alcohol treatment on the total distance moved [F(2, 24.75) = 5.65, P = 0.01]. However, a Games‐Howell post hoc test demonstrates that this difference is between the two ethanol‐treated groups (P = 0.008). Neither alcohol group differed significantly from controls, despite a trend for reduced locomotion in the 1.0% ethanol group (P = 0.09). These findings suggest that locomotion is unlikely to have an impact on social behavior in our analyses.
Figure 2.
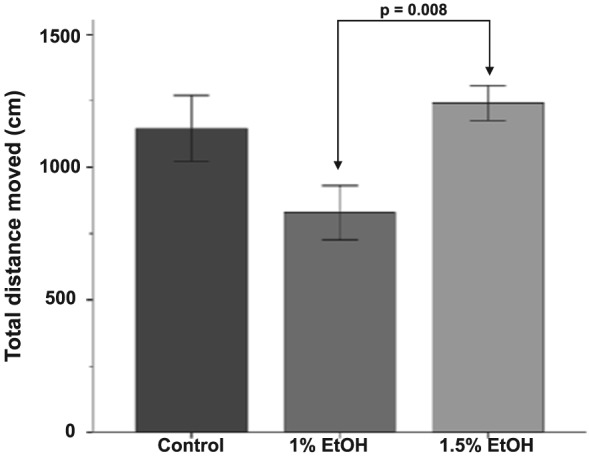
In 3‐week‐old zebrafish, the total distance moved by differs only between alcohol‐treated fish. Bars represent the total distance moved over the entire 30‐minute behavioral session. Mean ± SEM are shown. Sample sizes are as follows: Control (n = 16); 1% EtOH (n = 12) and 1.5% EtOH (n = 18). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. Note there was no statistical difference between controls and either alcohol treatment
In the larval assay, the test fish and conspecifics were separated from one another by a fixed transparent barrier and, during habituation, they were visually separated by a removable opaque insert. We first measured the percentage of time the larvae spent in each zone during habituation (Fig. 3). With the exception of zone 4, which is smaller than the others, zebrafish exposed to either 1.0% or 1.5% ethanol spent similar amounts of time in each zone. This larval social behavior has been described as relying solely on visual stimuli (Dreosti et al. 2015). Therefore, we were surprised that control fish spent substantially more time in the zone closest to the conspecifics, relative to any other zone, despite the presence of an opaque barrier (Fig. 4). Because the barriers between the fish are not water tight, this finding suggests that a nonvisual cue provides a weak preference for the zone nearest the conspecifics in control fish.
Figure 3.
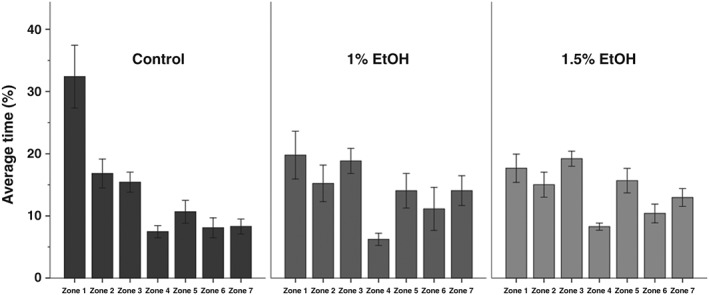
During habituation ethanol exposed, 3‐week‐old zebrafish display no preference for zone 1. Bars represent the average percentage of time 3‐week‐old zebrafish spend in all zones during the first 15 minutes of the behavioral session. Zone 1 is the zone closest to the live shoal, while zone 7 is the furthest from the live shoal. Mean ± SEM are shown. Sample sizes are as follows: Control (n = 16); 1% EtOH (n = 12) and 1.5% EtOH (n = 18). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization
Figure 4.
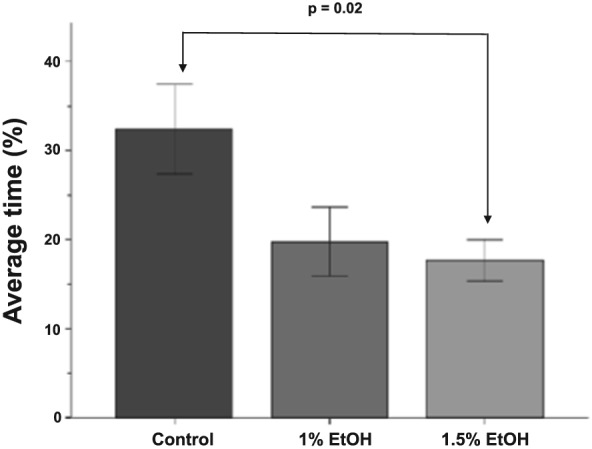
During habituation, untreated 3‐week‐old zebrafish spend more time in zone 1. Bars represent the average percentage of time 3‐week‐old zebrafish spend zone 1 during the last 15 minutes of the behavioral session. Zone 1 is the zone closest to the live shoal, while zone 7 is the furthest from the live shoal. Mean ± SEM are shown. Sample sizes are as follows: Control (n = 16); 1% EtOH (n = 12) and 1.5% EtOH (n = 18). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization
We used repeated measures ANOVA to determine if there were differences within these data. There was a significant zone effect [F(2.43, 104.59) = 14.19, P < 0.001] and a nonsignificant main effect of Alcohol treatment [F(2, 43) = 0.71, P = 0.50]. Importantly, there was a significant Zone × Alcohol treatment interaction [F(4.87, 104.59) = 2.63, P = 0.03]. While post hoc analyses cannot be performed on repeated measures ANOVAs, the data show that embryonic alcohol exposure affected the time spent across zones.
To specifically test if alcohol exposure ablated the weak social response to nonvisual stimuli, we compared the time spent in zone 1 during habituation across treatment groups (Fig. 4). There was a significant effect of Alcohol treatment on the percentage of time spent in zone 1 during habituation [F(2, 43) = 4.56, P = 0.02]. Tukey's HSD post hoc test revealed a significant difference between control fish (M = 32.41, SD = 20.18) and fish treated with 1.5% alcohol (P = 0.02, M = 17.67, SD = 9.66). Despite a strong trend, there was no significant difference between control fish and those treated with 1% alcohol (P = 0.08, M = 19.77, SD = 13.32). The nature of the cue being used by the control fish is currently unknown. However, our findings suggest that exposure to alcohol may affect the acquisition or integration of this unknown cue.
We next examined the time spent in all zones during the stimulus presentation to determine the effect embryonic alcohol exposure had on the social preferences of larval zebrafish [Fig. 5(a)]. All groups behaved nearly identically, spending the vast majority of the time in the zone nearest the conspecifics. Consistent with this, repeated measures ANOVA demonstrated a significant zone effect [F(1.60, 68.87) = 168.87, P < 0.001]. There was no significant main effect of Alcohol treatment [F(2, 43) = 1.7, P = 0.20] or interaction effect between Zone and Alcohol treatment [F(3.20, 68.87) = 0.50, P = 0.70]. Directly comparing the time spent in zone 1, we find no differences across groups [F(2, 43) = 0.3, P = 0.74] [Fig. 5(b)]. Taken together, these data suggest that embryonic alcohol exposure does not affect the strong social preference to a visible group.
Figure 5.
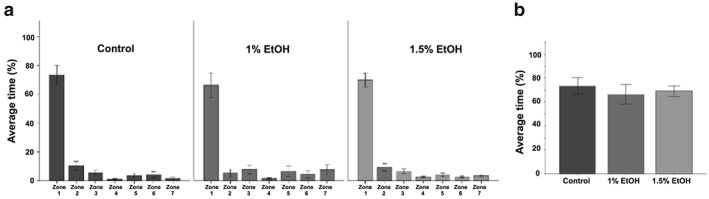
Embryonic alcohol exposure does not impair the social response of 3‐week‐old zebrafish. Mean ± SEM are shown. Sample sizes are as follows: Control (n = 16); 1% EtOH (n = 12) and 1.5% EtOH (n = 18). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. (a) Bars represent the average time 3‐week‐old zebrafish spent in each zone during stimulus presentation. Zone 1 is the zone closest to the live shoal. The alcohol concentration is shown below each graph. Note that fish from all groups spend the most time in the zone closest to the shoal. (b) Bars represent time average time spent in zone 1. Note that fish from all groups spend the most time in zone 1 when the stimulus is visible
Sixteen‐week‐old zebrafish (adult) behavior
Previous research has shown that when a single adult zebrafish can see either a group of live fish or computer animations of zebrafish, it will move towards the group (Qin et al. 2014). The single fish will approach the group and will remain within close proximity of the group (Fernandes et al. 2015), a behavior called a shoaling response. We characterized the average distance between the same test fish that had been assayed as larva and a shoal across each minute of the behavioral assay (Fig. 6). During the first 10 minutes (habituation), the test fish cannot see the shoal, while the shoal is visible (stimulus) for the second 10‐minute interval. In all groups, fish swim back and forth across the tank during habituation, resulting in an average distance approximating the middle of the tank (Figs 6 & 7). In all groups, fish move rapidly towards the stimulus once it becomes visible (Fig. 6). However, the ethanol‐exposed groups do not move as close to the shoal and fail to maintain this proximity (Fig. 6).
Figure 6.
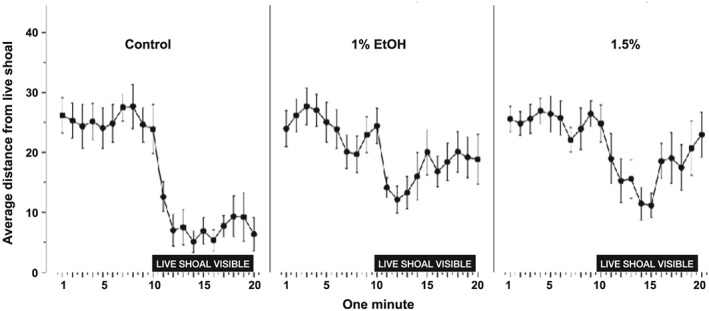
Embryonic ethanol exposure blunts the shoaling response in adult zebrafish. Average distance between the adult experimental fish and the live shoal plotted for 1‐minute intervals of the 20‐minute behavioral session. Mean ± SEM are shown. Sample sizes are as follows: Control (n = 12); 1% EtOH (n = 12) and 1.5% EtOH (n = 11). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. The horizontal bar above the x‐axis depicts timeline during which the live shoal is visible to the experimental subjects. The alcohol concentration is shown above the graphs
Figure 7.
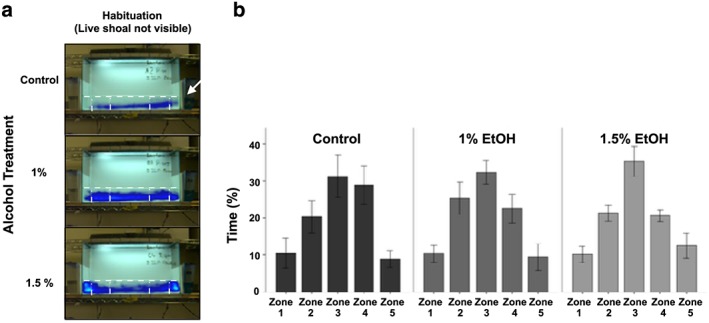
Embryonic alcohol exposure does not alter locomotor activity in adult zebrafish. (a) Representative heat maps. Frequency of a specific position is represented by a color (Version 11.5 XT, Noldus Info Tech, Wageningen, the Netherlands). The live shoal is positioned on the right side of the tank (single arrow). (b) Bars represent the average percentage of time spent in all five zones while the live shoal was not visible during the first 10 minutes (habituation). Mean ± SEM are shown. Sample sizes are as follows: Control (n = 12); 1% EtOH (n = 12) and 1.5% EtOH (n = 11). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. Zone 1 is the zone closest to the live shoal, while zone 5 is the furthest away from the live shoal
We used ANOVA to statistically analyze these data. We found a significant main effect of interval [F(7.95, 230.53) = 14.01, P < 0.0001] consistent with the rapid movement towards the stimulus upon presentation. There was a nonsignificant main effect of alcohol treatment [F(2, 29) = 2.82, P = 0.076]. Importantly, there was a significant interaction between interval and alcohol [F(15.90, 230.53) = 1.94, P = 0.02], consistent with the failure of the alcohol‐exposed fish to maintain proximity to the stimulus. Collectively, these data suggest that embryonic alcohol exposure blunts the shoaling response of adult zebrafish (Fig. 6).
To quantify this effect of ethanol on shoaling, we first compared the reduction in the distance with the live shoal once it is visible (average distance during stimulus presentation minus average distance during habituation) (Fig. 8). In this analysis, a larger negative value indicates a more robust response to the live shoal. There was a significant effect of Alcohol treatment on the change in distance [F(2, 32) = 6.94, P = 0.003). Tukey's HSD post hoc analysis demonstrated that control fish (M = −17.62, SD +/− 8.11) had a significantly stronger response to the live shoal than fish exposed to 1% alcohol (M = −7.19, SD = 6.71, P = 0.005) or 1.5% alcohol (M = 8.10, SD = 7.79, P = 0.013).
Figure 8.
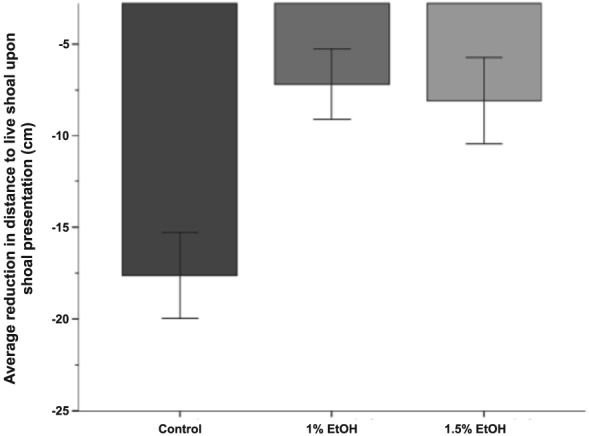
Embryonic alcohol exposure blunts the response to a social stimulus. Bars represent the difference between the distance fish where from the live shoal before and after the live shoal is visible. Larger negative values suggest a stronger response to the conspecifics. Mean ± SEM are shown. Sample sizes are as follows: Control (n = 12); 1% EtOH (n = 12) and 1.5% EtOH (n = 11). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. Note the diminished response caused by embryonic alcohol exposure
To more directly measure this shoaling response across groups, we quantified the amount of time a fish spent in each zone [Fig. 9(a)]. During habituation, all groups spent similar amounts of time in each zone [Fig. 7(b), note that not all zones are equal sized, see Materials and methods section for details]. A one‐way ANOVA showed that there was no significant effect of alcohol while a repeated measure ANOVA demonstrated that there was no interaction effect of alcohol by zone. Collectively, these data demonstrate that alcohol has no significant effect on the swimming behavior of zebrafish during habituation.
Figure 9.
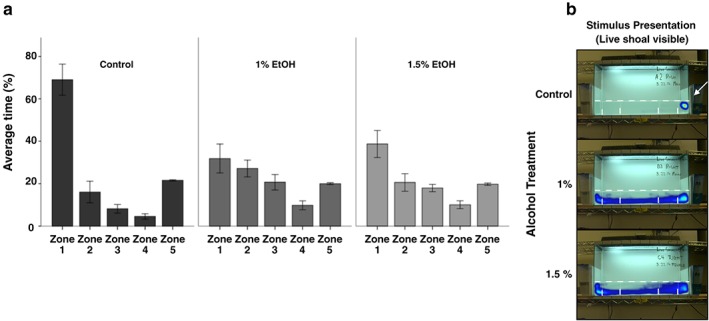
Embryonic alcohol significantly reduces the time fish spend in close proximity to a social group. (a) Bars represent the time spent in each of the five zones during last 10 minutes of the behavioral session. (b) Representative heat maps. Frequency of a specific position are represented by a color (Version 11.5 XT, Noldus Info Tech, Wageningen, the Netherlands). The live shoal is positioned on the right side of the tank (single arrow). Mean ± SEM are shown. Sample sizes are as follows: Control = 12; 1% = 12) and 1.5% = 11. Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. Zone 1 is the zone closest to the live shoal, while zone 5 is the furthest away from the live shoal. Note the significant difference in the amount of time control fish spend in zone 1 compared with alcohol‐treated fish
Upon shoal presentation, the distribution of time spent in each zone becomes highly skewed to the side of the tank adjacent to the shoal especially in untreated fish [Fig. 9(a) (b)]. We used repeated measures ANOVA to test for differences in these data. There was a significant main effect of zone and alcohol treatment [F(1.47, 47.02) = 33.12, P < 0.001 and F(2, 32) = 4.94, P = 0.013, respectively]. Most importantly, for the current study, there was a significant interaction between alcohol treatment and zone [F(2.94, 47.02) = 6.32, P = 0.001]. These data demonstrate that embryonic alcohol exposure alters the percentage of time spent across the zones of the testing arena. To further assay the shoaling response, we focused on the zone closest to the shoal. There was no difference in the length of time it takes the test fish to enter zone 1 following shoal presentation [F(2, 32) = 0.61, P = 0.55] (Fig. 10). Thus, embryonic alcohol exposure does not alter the initial response to the shoal or the ability to respond to the shoal.
Figure 10.
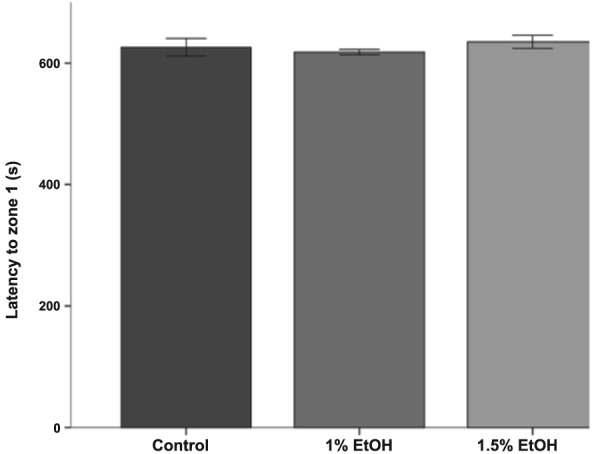
Embryonic alcohol exposure does not impair the immediate response to a social stimulus in adult zebrafish. Bars represent the time fish take to reach the zone 1 to the live shoal when the shoal is visible. The time is measured from start of the track. Mean ± SEM are shown. Sample sizes are as follows: Control = 12; 1% = 12) and 1.5% = 11. Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. Note fish from all groups reach the zone closest to the live shoal (zone 1) within a similar time frame
All groups spent the plurality of their time in the 5‐cm zone closest to the stimulus [Fig. 9(a)]. However, while control fish spent approximately 69% of the time in this small zone, fish exposed to 1% or 1.5% ethanol embryonically only spent 32% and 39% of their time in this same zone, respectively [Figs 9(a) & 11]. An ANOVA analysis demonstrated that there were significant differences in the time spent these groups spent in zone 1 [F(2, 32) = 8.44, P = 0.001]. A Tukey's post hoc analysis demonstrated that control fish spent significantly more time in zone 1 than did fish exposed to 1% (P = 0.001) or 1.5% ethanol (P = 0.011). Thus, a brief exposure to 1% or 1.5% ethanol in the embryo medium blunts the shoaling response with no apparent dosage effect.
Figure 11.
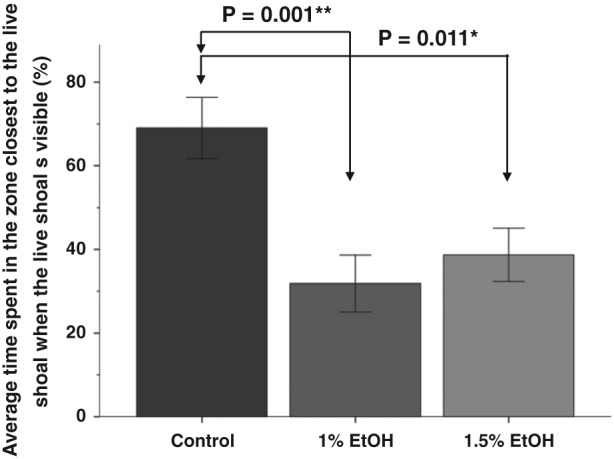
Embryonic ethanol exposure significantly reduces the time spent near a social group. Bars represent the average percentage of time spent in the zone closest to the live shoal while the live shoal was visible, during the last 10 minutes of the behavioral session. Mean ± SEM are shown. Sample sizes are as follows: Control (n = 12); 1% EtOH (n = 12) and 1.5% EtOH (n = 11). Embryonic alcohol exposure was conducted from 24 to 26 hours post‐fertilization. Note significant difference in the time spent close to conspecifics between treated and untreated fish
Discussion
There are a growing number of behavioral assays in zebrafish that are relevant to the study of FASD (Burton et al. 2017). Social deficits are common in FASD (Kelly, Day & Streissguth 2000; Rasmussen et al. 2011; Parker et al. 2014; Fernandes et al. 2015b). Here, we tested if these early social behaviors are disrupted by ethanol exposure and, by extension, how related these early behaviors are to adult shoaling. In sum, we find that, unlike the adult shoaling behavior, these early social behaviors are largely intact in ethanol‐exposed zebrafish. Our results demonstrate that this early social behavior is not sufficient for the development of adult shoaling responses. Whether the adult shoaling behavior is a consolidation of this early social behavior with other behaviors or is distinct from the early social behavior remains to be determined.
Adult behavior
Consistent with previous results (Fernandes & Gerlai 2009; Buske & Gerlai 2011; Parker et al. 2014; Fernandes et al. 2015a, b), we found that 1% ethanol severely blunts the shoaling response in adult zebrafish. The overall distance traveled and the initial response to the shoal were not changed in the ethanol groups, relative to controls. These findings further support previous conclusions (Fernandes & Gerlai 2009; Fernandes et al. 2015a, b) that the ethanol treatment is disrupting the shoaling behavior per se, not vision or motor control, both of which are involved in shoaling. The neurocircuitry underlying social behavior in other fish has been characterized (O'Connell & Hofmann 2011). Thus, it will be of great interest to determine the effects of early ethanol exposure on this circuitry in zebrafish.
Despite the social impairment, ethanol‐exposed zebrafish still spend more time in the zone closest to the shoal. Earlier studies utilizing a similar design to ours observed a dose response to concentrations of ethanol that varied from 0.25% to 1% (Fernandes & Gerlai 2009; Fernandes et al. 2015a). We found no further disruption to shoaling in zebrafish exposed to 1.5% ethanol, relative to 1% ethanol. The nature of this ethanol‐insensitive response to the shoal is unknown. It is possible that 1% ethanol maximally disrupts the social network, although analyses with doses of ethanol higher than 1.5% would be required to directly test this. Alternatively, zebrafish will approach novel stimuli (Saif et al. 2013). This residual behavior may reflect this response to a stimulus in general (i.e. not a social response but an approach response). Notably, characterization of the zebrafish social neurocircuitry along with analysis of how ethanol may disrupt responses to novel objects would help shed light on this question.
It is clear that adult shoaling behavior is exquisitely sensitive to ethanol‐induced disruption. These deficits are observed in response to computer‐animated shoals (Fernandes & Gerlai 2009; Fernandes et al. 2015a, b) and live shoals (Parker et al. 2014). Deficits can also be observed in the cohesiveness of fish within a live shoal (Buske & Gerlai 2011; Parker et al. 2014). Both the Tubingen (Parker et al. 2014) and AB (Fernandes & Gerlai 2009; Buske & Gerlai 2011; Fernandes et al. 2015a, b) strains of zebrafish are sensitive to ethanol‐induced shoaling defects. Therefore, zebrafish analyses will aid in understanding the mechanism that results in social deficits in FASD. Our analyses required an average per group sample size of 12 fish, about half that of previous characterizations, this should aid in these mechanistic, particularly genetic, analyses where large group sizes may be difficult to obtain.
Larval behavior
Our larval analyses were modeled on the initial characterization that social behavior in 3‐week‐old zebrafish required visible conspecifics (Dreosti et al. 2015). Therefore, it was a completely unexpected result that control fish spent more time in the zone closest to the live shoal during habituation. It is unclear why our analyses detect a weak, nonvisual, cue that promotes a putative social response. While there are some differences in the overall design of the testing chamber between these analyses, we think the differential response across studies is likely to be based on procedure. In their experiment, Dreosti et al. (2015) added the conspecifics after 15 minutes and tested the requirement for vision by performing analyses in the dark. We housed the live shoal behind opaque dividers, with the lights on, throughout habituation. Research in mice has shown that prenatal alcohol exposure impairs odor discrimination (Akers et al. 2011). Thus, in our experiment, it is possible that over time nonvisual, potentially chemical cues accumulated that were subsequently only detected by control fish. Alternatively, this weak response may be attenuated in the dark. Redesign of our current testing chamber will be required to distinguish between these possibilities.
Our second surprising result came when we found that ethanol‐exposed and control larva spent the same amount of time in the zone closest to the conspecifics during presentation [Fig. 5(a)]. Thus, the exact same fish show a robust social response at 3 weeks of age but a highly blunted social behavior as adults. This result demonstrates that the social behavior at larval stages and at adulthood is not equivalent and begs the question, why does the same alcohol exposure have a different effect on social preferences at different ages?
At this time, the reason for the different effects of embryonic alcohol exposure on social behavior at various ages is unknown. Shoals are thought to provide members with opportunities to forage, spawn and feed (Pitcher 1983). Perhaps different pressures are driving social behavior at the different ages. At the larva stage, fish are not sexually mature and exhibit no obvious external sexual dimorphisms. In adulthood, being in a group with other sexually mature fish has obviously fitness advantages. It is possible that the various motivational inputs into the social behavior network are differentially sensitive to ethanol. Collectively, our findings will allow us to further dissect social behavior both as it relates to FASD and as it develops over time.
Fernandes Y., Rampersad M., Jones E. M., and Eberhart J. K. (2019) Social deficits following embryonic ethanol exposure arise in post‐larval zebrafish, Addiction Biology, 24, 898–907. doi: 10.1111/adb.12649.
References
- Abel EL (1996) Fetal Alcohol Syndrome: From Mechanism to Prevention. CRC Press: Boca Raton, FL. [Google Scholar]
- Akers KG, Kushner SA, Leslie AT, Clarke L, van der Kooy D, Lerch JP, Frankland PW (2011) Fetal alcohol exposure leads to abnormal olfactory bulb development and impaired odor discrimination in adult mice. Mol Brain 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JM, Oliveri AN, Zhang C, Frazier JM, Mackinnon S, Cole GJ, Levin ED (2015) Long‐term behavioral impairment following acute embryonic ethanol exposure in zebrafish. Neurotoxicol Teratol 48:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotta J, Barnett JA, Hancock L, Saszik S (2004) Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol 26:737–743. [DOI] [PubMed] [Google Scholar]
- Burton DF, Zhang C, Boa‐Amponsem O, Mackinnon S, Cole GJ (2017) Long‐term behavioral change as a result of acute ethanol exposure in zebrafish: evidence for a role for sonic hedgehog but not retinoic acid signaling. Neurotoxicol Teratol 61:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Gerlai R (2011) Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol Teratol 33:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvan MJ III, Loucks E, Weber DN, Williams FE (2004) Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. Neurotoxicol Teratol 26:757–768. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2012) Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 61:517–522. [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius‐Spencer M, Lee L‐C, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin‐Allsopp M (2016) Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ 65:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti E, Lopes G, Kampff AR, Wilson SW (2015) Development of social behavior in young zebrafish. Front Neural Circuits 9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Buckley DM, Eberhart JK (2017) Diving into the world of alcohol teratogenesis: a review of zebrafish models of fetal alcohol spectrum disorders. Biochem Cell Biol 15:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R (2009) Long‐term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res 33:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M, Gerlai R (2015a) Embryonic alcohol exposure impairs the dopaminergic system and social behavioral responses in adult zebrafish. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M, Gerlai R (2015b) Impairment of social behaviour persists two years after embryonic alcohol exposure in zebrafish: a model of fetal alcohol spectrum disorders. Behav Brain Res 292:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M, Jia J, Gerlai R (2015) The effect of the number and size of animated conspecific images on shoaling responses of zebrafish. Pharmacol Biochem Behav 139 Pt B:94–102. [DOI] [PubMed] [Google Scholar]
- Finer LB, Zolna MR (2016) Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 374:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP (2000) Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol 22:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Am J Anat 203:253–310. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW (2007) Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev 31:192–201. [DOI] [PubMed] [Google Scholar]
- Kully‐Martens K, Denys K, Treit S, Tamana S, Rasmussen C (2012) A review of social skills deficits in individuals with fetal alcohol spectrum disorders and prenatal alcohol exposure: profiles, mechanisms, and interventions. Alcohol Clin Exp Res 36:568–576. [DOI] [PubMed] [Google Scholar]
- Lovely CB, Nobles RD, Eberhart JK (2014) Developmental age strengthens barriers to ethanol accumulation in zebra. Alcohol 48:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul‐Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE (2014) Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 134:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy N, Wetherill L, Lovely CB, Swartz ME, Foroud TM, Eberhart JK (2013) Pdgfra protects against ethanol‐induced craniofacial defects in a zebrafish model of FASD. Development 140:3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell LA, Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 519:3599–3639. [DOI] [PubMed] [Google Scholar]
- Parker MO, Annan LV, Kanellopoulos AH, Brock AJ, Combe FJ, Baiamonte M, Teh M‐T, Brennan CH (2014) The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development. Prog Neuropsychopharmacol 55:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Evans AM‐D, Brock AJ, Combe FJ, Teh M‐T, Brennan CH (2016) Moderate alcohol exposure during early brain development increases stimulus‐response habits in adulthood. Addict Biol 21:49–60. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ (1983) Heuristic definitions of fish shoaling behaviour. Anim Behav 31:611–613. [Google Scholar]
- Qin M, Wong A, Seguin D, Gerlai R (2014) Induction of social behavior in zebrafish: live versus computer animated fish as stimuli. Zebrafish 11:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C, Becker M, McLennan J, Urichuk L, Andrew G (2011) An evaluation of social skills in children with and without prenatal alcohol exposure. Child Care Health Dev 37:711–718. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP (1999) Behavioral and psychosocial profiles of alcohol‐exposed children. Alcohol Clin Exp Res 23:1070–1076. [PubMed] [Google Scholar]
- Saif M, Chatterjee D, Buske C, Gerlai R (2013) Sight of conspecific images induces changes in neurochemistry in zebrafish. Behav Brain Res 243:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC. eds. (1996) Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press: Washington, D.C. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF (1991) Fetal alcohol syndrome in adolescents and adults. JAMA 265:1961–1967. [PubMed] [Google Scholar]
- Westerfield M (1993) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). University of Oregon Press: Eugene. [Google Scholar]
- World Health Organization … (2014) Global status report on alcohol and health. Geneva, Switzerland: World Health Organization.


