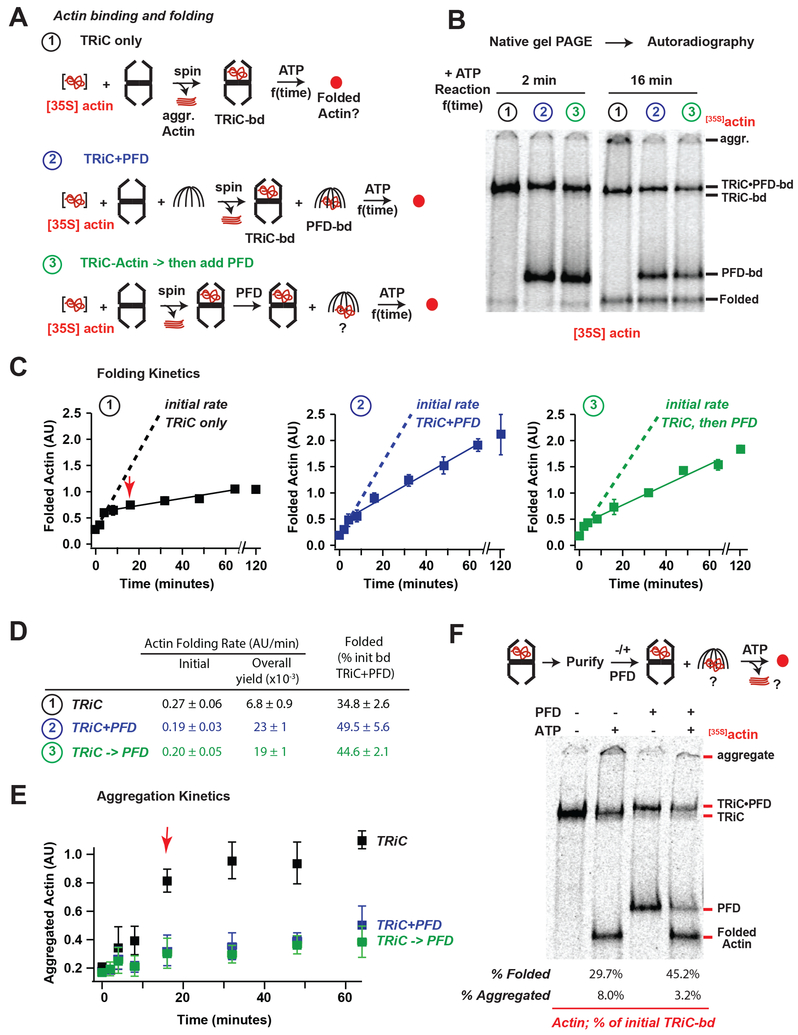
Figure 3. Prefoldin acts on the TRiC-substrate complex to increase folding processivity.
(A) Actin folding assays: ①TRiC alone, ②TRiC and PFD during initial Actin binding, and③PFD added after initial Actin binding to TRiC. Folding was initiated by raising temperature to 37 °C and ATP addition. (B) Representative autoradiograms of the 2 minute and 16 minute timepoints of folding, note appearance of aggregates in TRiC alone sample at 16 min. (C) Plots of quantified Actin folding for each reaction, dotted lines represent linear fits to first 3 time points (0–4 min), solid lines represent linear fits to later time points (4–64 min), the average amount of folded actin for the final 3 time points of TRiC alone is set as 1 in each replicate, error bars represent SEM. (D) Initial and steady state folding rates for each reaction. (E) Plot of amount of aggregated Actin with minimal migration into the native gels, note significant decrease in folding rates for TRiC alone reaction correlate with appearance of aggregated Actin (red arrow). (F) Actin bound to TRiC was purified from unbound Actin and folding assays were carried out in the absence or presence of PFD, strikingly Actin transferred from TRiC to PFD both in the absence and presence of ATP