Abstract
Temperature and salinity effects on marine diatom species growth has been studied extensively; however, their effect on arsenic (As) biotransformation has been imprecise. This study reports the growth, and As biotransformation and speciation patterns at various temperatures and salinities of six marine diatom species: Asteroplanus karianus, Thalassionema nitzschioides, Nitzschia longissima, Skeletonema sp., Ditylum brightwellii, and Chaetoceros didymus. The growth rate and As biotransformation potentials of these species during three weeks of culture in f/2 based medium were significantly affected by wide temperature (0–35 °C) and salinity (0.3–50‰) ranges. Growth and As biotransformation were higher at optimum temperatures of 10–25 °C, and salinity of 10–35‰, whereas growth and arsenic biotransformation were lower at <5 °C and 5‰ and >25 °C and 35‰, respectively. The results showed that As(V) to As(III) biotransformation differed significantly (p < 0.05) between day 10 and 17. At optimum temperature and salinity levels, the cell size and As biotransformation were higher for all the species. A conceptual model on temperature and salinity effects on growth and As uptake and biotransformation mechanisms by these species has been proposed based on the findings of this study.
Subject terms: Biochemistry, Marine chemistry
Introduction
Arsenic (As), a toxic metalloid, is mostly found in soil, freshwater, and marine ecosystems. Anthropogenic activities, together with natural sources, contribute to increased contamination of surface and ground water. As has four oxidation states, such as arsenate (As(V)), arsenite (As(III)), arsenic (As°), and arsine (As(-III)), each of which comprises different physico-chemical characteristics1. Biotransformation of As species by aquatic organisms is a complex mechanism with different toxicity levels2. The toxicity of different As forms as determined by the 50% lethal dose (LD50) follow the order: As(III) (14) > As(V) (20) > monomethylarsonate (MMAA(V)) (700–1800) > dimethylarsinate (DMAA(V)) (700–2600) > arsenocholine (ArsC) (>6500) > arsenobetaine (ArsB) (>10,000)3. Inorganic arsenic (iAs) is more toxic than organoarsenic (orgAs); however, the toxicity on aquatic organisms depends on the As concentration and its speciation4. Microalgae reduces the toxic effect of iAs through several processes, including As(V) reduction, As(III) oxidation, methylation, conversion to arsenosugars/arsenolipids, complex formation of As(III) with glutathione and phytochelatins, cell surface binding, and excretion from the cell5. In marine environments, algal species uptake iAs (As(V) and As(III)) in the form of arsenic and biotransform it into methylated arsenic (methylAs) species and/or arsenosugars (AsS), such as orgAs species6. The concentration of iAs and methylAs species varies in marine microalgae cells, whereas As biomethylation produces methylAs species from iAs. This phenomenon is determined by the type of microalgae species and their characteristics because different species have various biotransformation abilities7. In the aquatic food chain, micro or macro algae, as members of lower trophic levels, take up As content more actively than higher trophic members8. According to Sanders et al.9, microalgae, as primary producer, accumulate As(V) from surrounding sea water and reduced it to As(III), and this biotransformation process elucidates the As(III) and As(V) ratio in marine aquatic environments. The chemical formation of As(III), MMAA) and dimethylarsinate (DMAA) are actively associated with primary productivity10 in marine water where microalgae play a pioneer role in the formation of such As species11.
However, growth of microalga, particularly diatom species, and their As biotransformation mechanisms are influenced by several factors, including constituency of nutrient medium12, concentration of As species2,13, pH14–16, light intensity2,17, temperature18,19, salinity20,21, and length of exposure period22. In this study, we focused on the two essential environmental factors, temperature and salinity, which regulate the growth and physicochemical metabolism in microalgae in marine ecosystems. The nutritional properties of microalgal species are stimulated by salinity and temperature variations in the environment23. Moreover, temperature plays a significant role on the growth rate24, chemical composition25, and metabolic processes of marine diatom species. Biochemical and physiological metabolism, such as growth, photosynthesis, and As accumulation and biotransformation are influenced by salinity in marine ecosystems. Although, adaptation and tolerance of microalgae varies between species, diatoms are directly or indirectly affected by salinity where the ion composition is one of the growth factors26,27. Many algal species can grow in a wide range of temperatures and salinities, exhibiting high tolerance to variation of such factors28.
The influence of temperature25,29–32 and salinity33–35 and their combined effect36,37 on the growth and development of marine diatoms have been studied in detail. However, limited information on the effect of temperature and salinity on As bioaccumulation, biotransformation, and speciation pattern, particularly by marine diatom species, is available. Moreover, the effects of salinity and temperature on cell sizes of marine diatom species and their interrelated influence on As biotransformation is also very limited in the literature. Therefore, this study aims to breech the research gap by investigating the influence of temperature and salinity on As biotransformation and speciation pattern by six marine diatom species. The interrelated influence of temperature, salinity, and cell size on As biotransformation was investigated. This study also highlighted the diatom species specifically associated with temperature- and salinity-dependent As biotransformation mechanisms, which may further provide important insight regarding As remediation processes.
Results and Discussion
Effects of temperature and salinity on the growth of marine diatom species
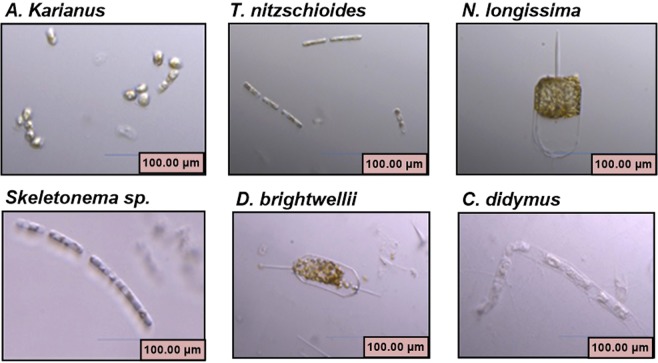
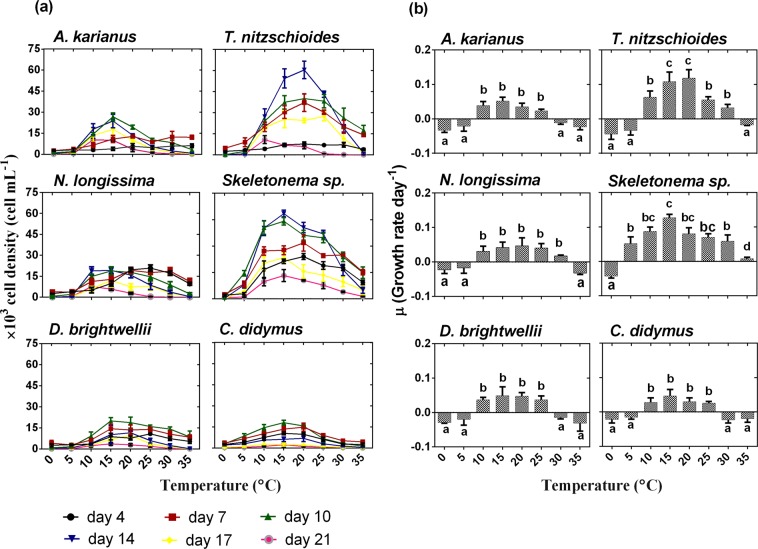
Temperature is a factor that can be controlled in microalgal cultivation and is a sensitive factor for algal growth and metabolic processes. Digital microscope images of six marine diatom species used in this study are shown in Fig. 1. The maximum cell density (cells mL−1) was recorded at temperatures between 10 and 25 °C for all the species (Fig. 2a). Among the six species, Thalassionema nitzschioides and Skeletonema sp. showed exceptional cellular growth capacities with wide temperature tolerances. All the species exhibited higher cell densities on day 10 and 14 during the 3 weeks of culture. On day 14, the maximum cell concentration was recorded for T. nitzschioides (cell density 60.4 × 103 cell mL−1/growth rate 0.11 ± 0.02 day−1 at 20 °C) and Skeletonema sp. (cell density 64.6 × 103 cell mL−1/growth rate 0.12 ± 0.01 day−1 at 15 °C) (Fig. 2a,b). This result possibly suggests that these temperatures are optimum for the growth of these two species. Except for T. nitzschioides and Skeletonema sp., the remaining diatom species showed a similar growth pattern at various temperatures on different culture days. On day 10 at 15 °C, Asteroplanus karianus, Nitzschia longissima, Ditylum brightwellii, and Chaetoceros didymus displayed higher cell growth with cell densities of 26.8 × 103 (growth rate 0.05 ± 0.01 day−1), 18.8 × 103 (growth rate 0.04 ± 0.02 day−1), 20.1 × 103 (growth rate 0.05 ± 0.03 day−1), and 18 × 103 cell mL−1 (growth rate 0.05 ± 0.02 day−1), respectively (Fig. 2a,b). The effects of temperature on algal species may vary depending on the species type, characteristics, and surrounding environment. Fujimoto et al.18 reported that the growth rate of a microalga (Selenastrum capricornutum) was higher when temperature was adjusted to 22 °C, whereas that of Microcystis viridis was higher at 30 °C. A positive correlation was observed between temperature and Nannochloris oculata growth in a temperature range of 20 to 25 °C38, below this temperature the growth rate decreased from 0.13 to 0.06 day−1 39. The optimum temperature for Scenedesmus sp. growth was found to be between 20 °C and 40 °C40–42. Algal species, such as C. vulgaris grow well at 30 °C; however, when the temperature was increased to 35 °C, the growth rate decreased to 17% and the species died at 38 °C39. Therefore, the growth of our experimental species was dependent on the capability to survive in certain temperature ranges, which is optimum for their growth.
Figure 1.
Images of six marine diatom species used in this study, captured using a digital microscope (KEYENCE, VHX-1000, Japan).
Figure 2.
Effect of temperature (°C) on the growth of marine diatom species, (a) cell density (cell mL−1), (b) growth rate day−1. Different lowercase letter in Fig. 2(b) indicates significant differences between temperature levels. Data are means ± SD (n = 3).
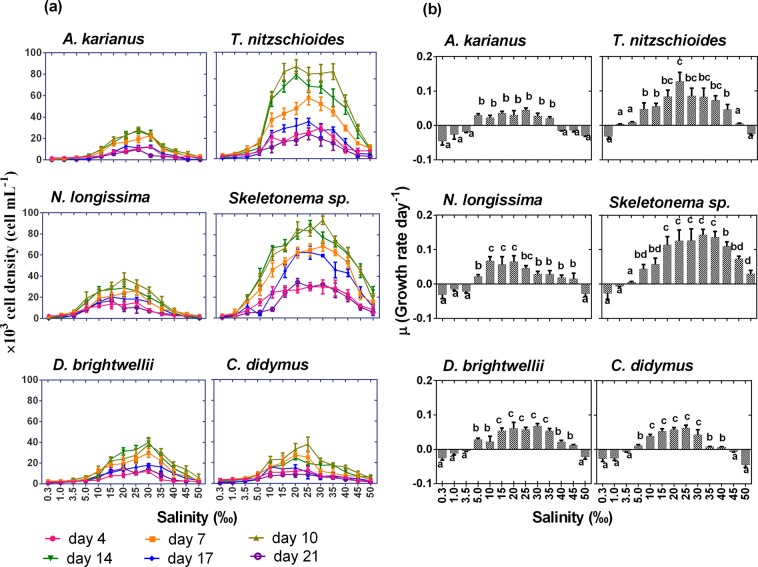
Salinity is an important factors that has significant effects on plant growth and their metabolic activities43. A wide range of salinity levels (0.3–50‰) was employed to observe the cell concentrations and growth rate per day of the marine diatoms in the culture vessels. All diatom species showed maximum cell concentrations on day 10 of incubation at salinities between 10 and 35‰ (Fig. 3a). Similarly, to the temperature treatment, T. nitzschioides and Skeletonema sp. showed higher salinity tolerances than the other experimental species. On day 10 of culture, the highest cell concentration of 69.2 × 103 cell mL−1 (growth rate 0.13 ± 0.03 day−1) at 20‰ and 94 × 103 cell mL−1 (growth rate 0.14 ± 0.12 day−1) at 30‰ salinity level was recorded for T. nitzschioides and Skeletonema sp., respectively (Fig. 3a,b). A similar maximum growth rate (0.06 ± 0.02 day−1) was observed for N. longissima, D. brightwellii, and C. didymus. A growth rate of 0.04 ± 0.01 day−1 with cell concentration of 28 × 103 cell mL−1 was observed for A. karianus at 25‰ salinity level (Fig. 3a,b). Different salinity tolerance levels and adaptation strategies by microalgal species largely depend on the group and environmental characteristics26. In natural environments, multiplication of marine microalgae generally occurred between days 5 to 7 of their growth stage and they could survive for at least 21 days depending on the species. The growth and salinity tolerance (low growth expectancy between <5‰ and >35‰) of the marine diatom species of the present study were also supported by several previous studies27,44,45.
Figure 3.
Effect of salinity (‰) on the growth of six marine diatom species, (a) cell density (cell mL−1), (b) growth rate day−1. Different lowercase letter in Fig. 3(b) indicates significant differences between salinity levels. Data are means ± SD (n = 3).
Effects of temperature and salinity on As biotransformation
In this study, the cultures were exposed to 0.1 µmol L−1 Na2HAsO4 as As(V) and 1 µmol L−1 NaH2PO4 as PO43− to influence the uptake mechanisms of the diatom species. Microalgae take up arsenic species in the form of As(V) using the phosphate pathway in the cell membrane owing to the physiochemical similarities between As(V) and PO4 3–12,46. Marine microalgae reduce the toxic effect of As(V) by transforming As(V) to As(III) inside the cell47,48. This phenomenon occurs by a reduction of two electrons of pentavalent arsenate to trivalent arsenite facilitated by thiol, such as glutathione49. The conversion of As(V) to As(III) and the following biomethylation to methylated arsenicals, e.g. DMAA and MMAA, by microalgae50 largely depends on the species growth capability and concentration of phosphate and arsenic in the culture medium.
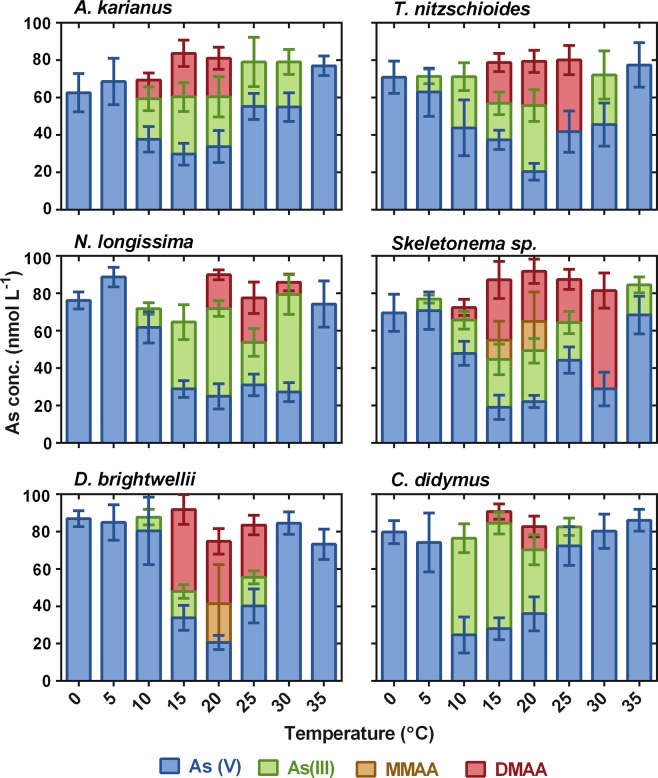
The potential of As biotransformation by six marine diatom species was investigated under various temperatures (0–35 °C) at day 10 of culture (Fig. 4). Except for T. nitzschioides and Skeletonema sp., none of the other microalgae showed biotransformation of As species at ≤5 °C and ≥35 °C. At 5 °C, As biotransformation was detected only for T. nitzschioides (62.9 ± 13 nmol L−1 of As(V) and 8.4 ± 4 nmol L−1 of As(III)) and Skeletonema sp. (As(V) = 73.7 ± 10 nmol L−1 and As(III) = 6.3 ± 2.1 nmol L−1), whereas Skeletonema sp. even biotransformed As at 35 °C (As(V) = 68.4 ± 10.1 nmol L−1 and As(III) = 16.1 ± 4.3 nmol L−1) (Fig. 4). All the species transformed As(V) to As(III) and the methylated forms of As species i.e. DMAA and/or MMAA at 15 and 20 °C. The reduction of As(V) to As(III) and/or DMAA was witnessed from 10–30 °C for A. karianus and N. longissima, 5–30 °C for T. nitzschioides, and 10–25 °C for D. brightwellii and C. didymus (Fig. 4). This phenomenon was observed for Skeletonema sp. even at a wide temperature range (5–35 °C). The above results indicated that all the diatom species of this study could grow and potentially biotransform toxic As(V) to As(III) with further methylation to form methylAs species at certain temperature ranges at their logarithmic growth phase.
Figure 4.
Effect of temperature (°C) on arsenic biotransformation processes (As(V) is reduced to As(III) and subsequent methylation to MMAA and DMAA) by six marine diatom species. Data are means ± SD (n = 3).
The present study elucidated the relationship between temperature on the growth of diatom species and As biotransformation. None of the species showed biotransformation at 0 °C, suggesting that at such low temperatures these species are unable to reduce As(V) to As(III) and the subsequent methylation to MMAA and DMAA. However, the presence of small amounts of MMAA and MMAA not only assist in iAs methylation, but also help the fast release of MMAA and DMAA when exposed to As(V)51. Similarly, at 35 °C, no As biotransformation occurred, except for Skeletonema sp. for growth and As biotransformation processes. However, As(V) was reduced to As(III) either for further methylation or efflux from the cell52 by D. brightwellii and C. didymus at 10–25 °C. The influence of temperature on As uptake mechanisms and speciation were very imprecise. However, several researchers exposed cultures to temperatures of 20–35 °C to investigate As uptake and metabolism by phytoplankton18,22,53, suggesting that high temperatures are important to reduce arsenic uptake. Cho et al.54 observed that maximum cellular growth of Chlorella ellipsoidea and Nannochloris oculata at 20 °C and 30 °C, respectively, but the study did not investigate the As uptake and biotransformation processes. These findings illustrated that As biotransformation depends on the specific species and their metabolism, in addition to adaptability to different temperature conditions.
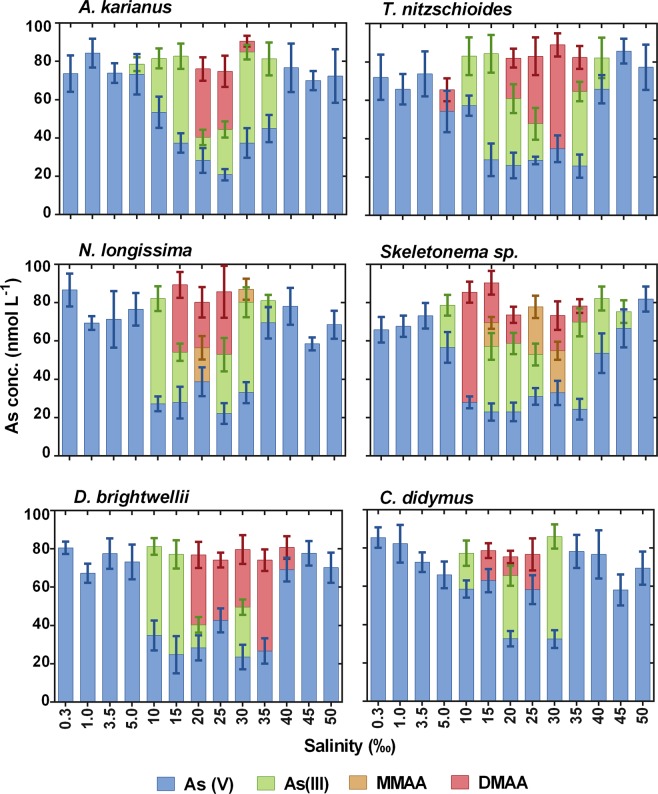
The biotransformation of As species and the subsequent methylation by six diatom species occurred well between salinities of 10 to 35‰ (Fig. 5). At low salinity levels (0.3–3.5‰), only As(V) was measured in the culture medium, indicating that at such low salinities all the species were unable to reduce As(V) to As(III) or methylated arsenicals. A 5‰ salinity was too low for cellular growth and As biotransformation of N. longissima, D. brightwellii, and C. didymus. At high salinity levels (45–50‰), no biotransformation was occurred, except for in Skeletonema sp. This species possesses a wide range of salinity tolerance (5–45‰) and could biotransform As(V) to As(III), even at a salinity of 45‰ (As(V) = 66.5 ± 9.8 nmol L−1 and As(III) = 8.8 ± 5.8 nmol L−1) (Fig. 5). This is might be because different species react differently in different salinities, which has been reported in several other studies44,45,55. This is an agreement a previous study indicating that Skeletonema sp. grow well under wide salinity conditions in the natural environment34,56,57. However, C. didymus showed As biotransformation under salinities between 10–30‰, whereas below 5‰ and above 35‰, the redox reaction, as well as methylation, was absent. This phenomenon may occur because (i) microalgal metabolism is affected by extreme high or low ion concentrations and (ii) certain metabolites required for cellular growth are depleted. In addition, salinity changes influenced changes in turgor pressure, which in turn modified the membrane thickness. The biochemical and biophysical processes of microalgal cells are possibly connected with the translocation rate of mobile charges located inside the membrane, which is regulated by the membrane thickness58. The redox reaction by microalgae generally occurs as a protection mechanism to avoid metalloid toxicity14, or as a supportive system for cellular growth in the mode of energy production59. Biotransformation activity by marine species may be influenced by different salinity conditions, as it has an obstructive effect on the central metabolic activity43.
Figure 5.
Effect of salinity (‰) on arsenic biotransformation processes (As(V) is reduced to As(III) and subsequent methylation to MMAA and DMAA) by six marine diatom species. Data are means ± SD (n = 3).
Effects of temperature and salinity on As speciation pattern: time dependency study
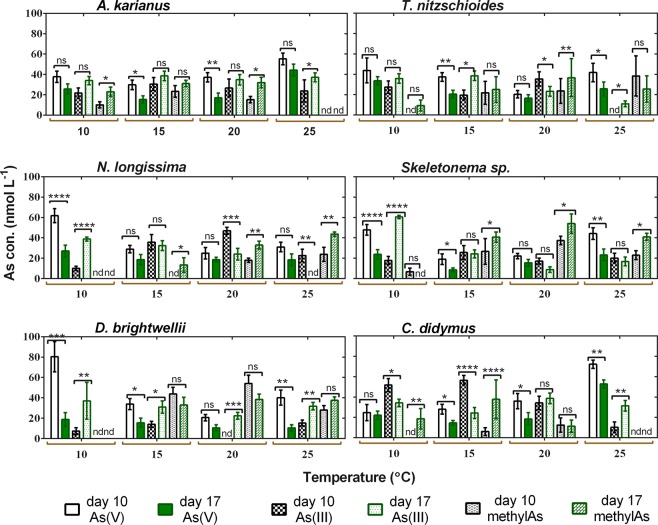
Arsenic speciation pattern by marine diatoms was observed at four different temperatures (10, 15, 20, and 25 °C) in a week i.e. on day 10 and 17 of culture (Fig. 6). These temperatures seemed optimum to all the species, as growth and As biotransformation were not properly observed for all the species below 10 °C and above 25 °C. The result indicated that in some cases, the biotransformation of As(V) to As(III) and subsequent methylated arsenicals was significantly different (p < 0.05) between day 10 and 17 of speciation. For example, on day 10 at 10 °C, A. karianus contained 37.7 ± 5.6, 21.7 ± 5.2, and 10.1 ± 3.1 nmol L−1 of As (V), As(III), and methylated As component (MMAA + DMAA), respectively, whereas on day 17 at the same temperature, these amounts were 25.7 ± 4.9, 33.9 ± 4.0, and 23 ± 4.4 nmol L−1 (Fig. 6). Regarding T. nitzschioides, the concentrations of As(V), As(III), and methylAs component were 43.8 ± 12.1, 27.4 ± 6.0, and 0 nmol L−1 on day 10 at 10 °C, whereas these amounts were 33.6 ± 4.0, 35.9 ± 4.8, and 9.5 ± 5.2 nmol L−1 on day 17 at the same temperature, respectively (Fig. 6). Other species, such as N. longissima, Skeletonema sp., D. brightwellii, and C. didymus showed significant As biotransformation from days 10 to 17. Lower As(V) concentrations on day 17 than on day 10 at each temperature indicated that As(V) decreased with time in the culture medium. The occurrence of As(III) and methylAs in the medium was mainly a biological reduction of As(V) to As(III). In addition, methylated arsenicals (MMAA and DMAA) were also found at higher concentrations on day 17 than that on day 10. The conversion efficiency of pentavalent arsenate (As(V)) to the reduced form of trivalent arsenite (As(III)) was considered to be the pioneer step for the methylation process60. As biotransformation by marine diatom species in this study revealed that they are capable of redox reactions, methylation, and excretion of As in the medium.
Figure 6.
Time-dependent As speciation patterns by six marine diatom species. As speciation was observed on day 10 and 17 of culture at various temperatures (°C). The star marks above the bars showed significant differences at *p ≤ 0.01, **p ≤ 0.001, and ***p ≤ 0.0001 levels between day 10 and 17 within the same As species. ‘ns’ indicates non-significant, ‘nd’ indicates not detected. Data are means ± SD (n = 3).
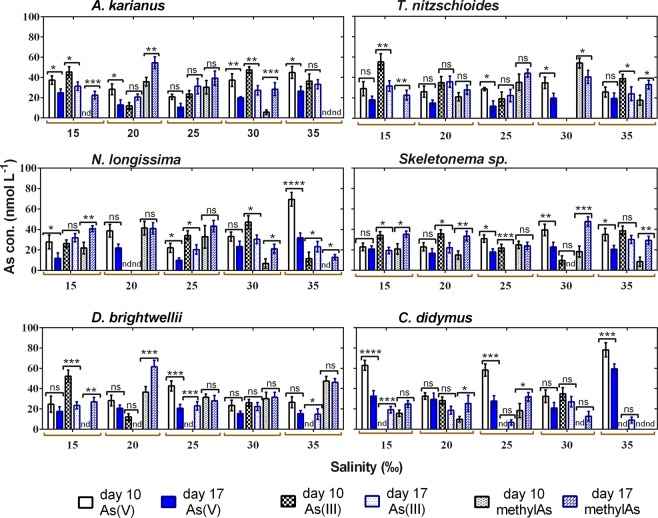
Similarly, to temperature, a time dependency study was conducted at five salinity levels (15, 20, 25, 30, and 35‰), and As speciation was recorded on day 10 and 17. At all salinities, the biological reduction of As(V) by marine diatom species was recorded between day 10 and 17 (Fig. 7). For example, at day 10 and 25‰ salinity condition in T. nitzschioides, the concentrations of As(V), As(III), and methylAs were 28.6 ± 1.6, 19.1 ± 6.8, and 35.2 ± 8.0 nmol L−1, whereas on day 17 at the same temperature these concentrations were 11.9 ± 5.2, 22.2 ± 6.1, and 44.2 ± 3.9 nmol L−1, respectively (Fig. 7). In some cases, at certain salinity conditions on day 10, more than 60% As(V) remained in the medium. For example, on day 10 at 35‰ salinity, N. longissima and C. didymus species contained 69.4 ± 6.7 and 76.6 ± 7.0 nmol L−1 of As(V), respectively (Fig. 7). However, C. didymus contained an As(V) concentration of 59.5 ± 4.8 nmol L−1 at day 17 at 35‰, which was the only exception found during the analysis of the data related to salinity-based time dependency, but is difficult to explain. As the regulation of As uptake is different for different species, it may be controlled by the intracellular As(V) concentration, and then its accumulation could be activated by its release from the cell5,61. As(V) uptake or sequestration is enhanced by the physio-biochemical structure in association with individual species type and characteristics62.
Figure 7.
Time-dependent As speciation pattern by six marine diatom species. As speciation was observed on day 10 and 17 of culture at various salinity (‰) levels. The star marks above the bars showed significant differences at *p ≤ 0.01, **p ≤ 0.001, and ***p ≤ 0.0001 levels between day 10 and 17 samples within the same As species. ns indicates non-significant, nd indicates not detected. Data are means ± SD (n = 3).
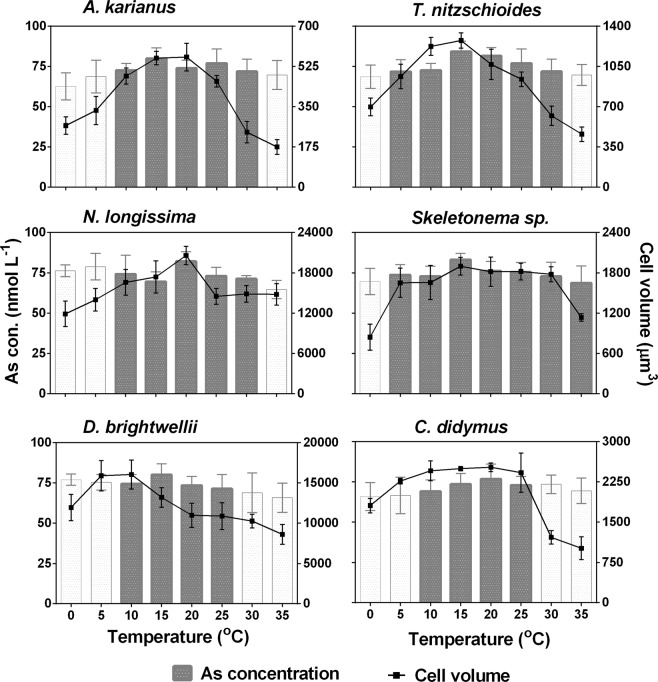
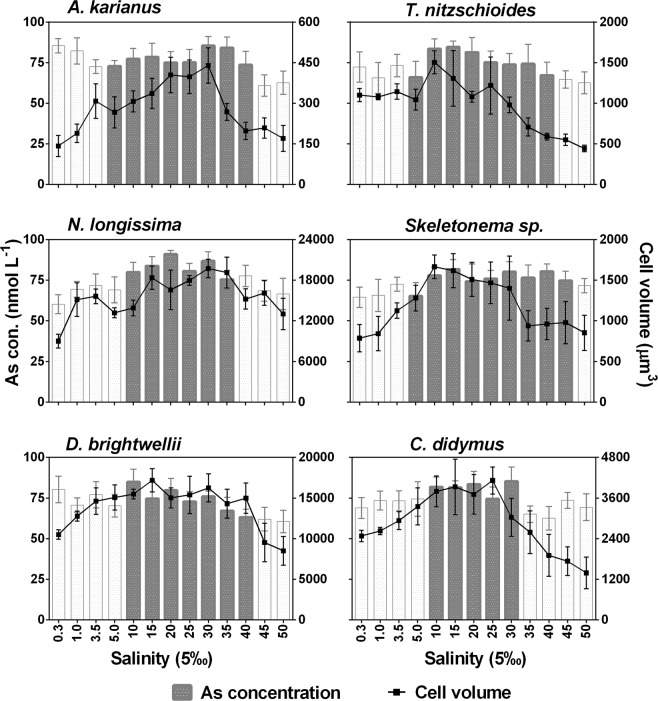
Interrelated influence of temperature, salinity, and cell size on As biotransformation
Cell surface area (µm2) of six diatom species was calculated at different temperatures (0–35 °C) and salinities (0.3–50%o) (Table S1, Appendix A: Supporting information). The maximum surface area of the cell was in the ranges of optimum temperature (10–25 °C) and salinity (10–35‰) for all the species. Below and above these temperature and salinity levels, individual diatom cells had a reduced surface area for adaptation to the adverse environment. The interrelation between cell volume (µm3) of each diatom species and As biotransformation potentials were evaluated (Figs 8, 9). The cell volume and As biotransformation were positively correlated in almost all of the cases. Cell size of the six diatom species in the descending order: N. longissima > D. brightwellii > C. didymus > Skeletonema sp. > T. nitzschioides > A. karianus. At the optimum temperature and salinity levels, all the species could biotransform As(V) to As(III) or MMAA or DMAA at a maximum cell volume. The diatom species were unable to biotransform As species at temperatures <5 °C and >30 °C, and salinity <5‰ and >35‰ with some variations (Figs 8, 9). The As biotransformation potentials of the six diatom species during temperature treatment was: Skeletonema sp. > T. nitzschioides > N. longissima > A. karianus > D. brightwellii > C. didymus, whereas during the salinity treatment the order was: Skeletonema sp. > T. nitzschioides > A. karianus > D. brightwellii > N. longissima > C. didymus.
Figure 8.
Relationship between cell size and As biotransformation of six marine diatom species at various temperature levels. Dark bars in the graph indicate As species biotransformation occurred, i.e. As(V) was reduced to As(III) and the subsequent methylation to MMAA and DMAA. White bars indicate no biotransformation occurred, i.e. only As(V) was detected in the culture medium. Data are means ± SD (n = 3).
Figure 9.
Relationship between cell size and As biotransformation of six marine diatom species at various salinity levels. Dark bars in the graph indicate As species biotransformation occurred, i.e. As(V) was reduced to As(III) and the subsequent methylation to MMAA and DMAA. White bars indicate no biotransformation occurred, i.e. only As(V) was detected in the culture medium. Data are means ± SD (n = 3).
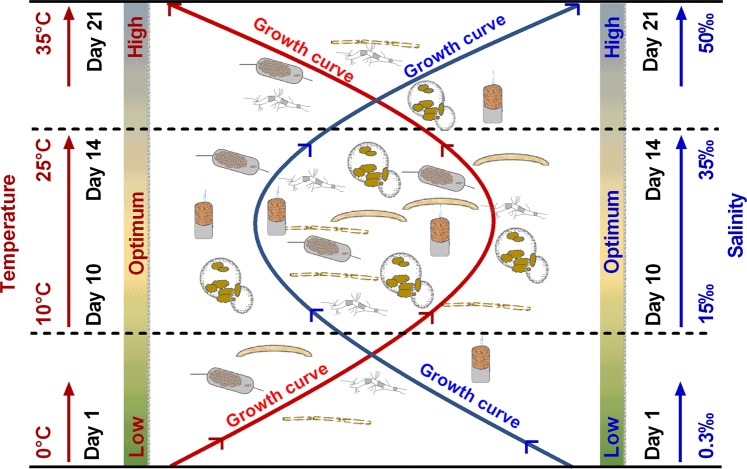
Conceptual model of growth and arsenic uptake mechanisms
A conceptual model on the effect of temperature and salinity on the growth and As uptake and biotransformation mechanisms by six marine diatom species was developed based on the findings of this study (Figs 10, 11). Microalgal growth is influenced by environmental conditions, such as temperature and salinity28,63,64, which are considered as crucial environmental factors during their culture. The effect of both temperature and salinity on the growth of six marine diatom species showed similar trends based on the culture day. The maximum cell density (cell m L−1) was recorded for all the diatom species on day 10 and 14 of culture for both temperature and salinity treatments. All the species showed optimum growth as cell density at temperatures between 10 and 25 °C and salinity levels between 10 and 35‰ (Figs 2a, 3a, 10). The temperature tolerance of algal species depends on rapid changes of temperature and the physicochemical state of algae exposed to extreme temperature before, during, and after a period of time65. Moreover, salinity influences the growth of the species regulated by osmoregulatory mechanisms, which further affects the physiological and biochemical process of algal species in marine environments.
Figure 10.
Conceptual model on effect of temperature and salinity on the growth of six marine diatom species.
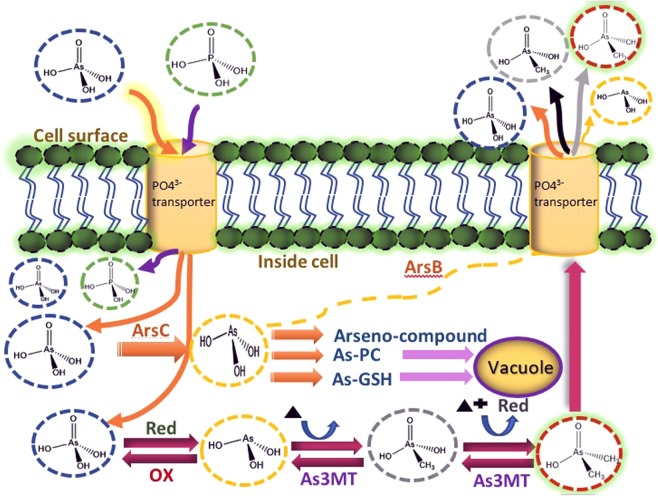
Figure 11.
Conceptual model on effect of temperature and salinity on arsenic uptake and biotransformation mechanisms by six marine diatom species based on this study.
In aquatic environments, As(V) accumulation and biotransformation to As(III) and subsequent methylation to MMAA and DMAA by photosynthetic microbial species e.g. algae and cyanobacteria50, is affected by environmental factors, such as temperature, salinity, pH, and nutrients. These factors control the algal biochemical composition and behaviour in natural aquatic ecosystems. These microbial species play a significant role in regarding biogeochemical cycling of As content by biotransformation in natural ecosystems48. As(V) and phosphate have similar physicochemical characteristics, and therefore algal species take up As(V) present in media via a phosphate transporter system66 and As(V) biotransformation occurs inside the cell67. The competitive uptake between As(V) and PO43− suggested a possible active toxic mode of As. Inside the cellular portion of ATP, As(V) replaced the phosphate groups and established an unstable ADP-As complex that in turn interfered with several physiological process, e.g. energy flow68. Furthermore, after As(V) uptake, it is either transformed to trivalent As and excreted into the medium or subsequently methylated to methyl arsenicals. The reduced metabolites As(III) readily released from the cell leading to reduced the toxicity of As(III) inside of the cell69,70. This process of biomethylation from iAs is regarded as the detoxification mechanism of aquatic algal species71. In addition, excretion of As(V), As(III), or the organic component could be regarded as a significant arsenic detoxification mechanism72.
As(V) toxicity is based on the competitive inhibition of protein, along with other enzymes that use phosphate, as well as oxidative phosphorylation. The reduction reaction of As(V) to As(III) occurs in the presence of thiols and/or dithiols because of its tendency to combine with biochemical components, such as protein and non-protein thiols73. However, As(III) is more toxic than As(V) because of its actions, similarly to soft metal with thiols. In addition, the As(III) bond with monothiol is comparatively weak and its high concentration depletes glutathione within the cell. It develops strong bonds with dithiols, which inactivates different important enzymes and receptors74. After As(V) uptake from surrounding marine water, As(V) is organized to form carbohydrate groups, which are further biosynthesized to orgAs species6. However, orgAs contained by marine microalgae are mostly AsS that are regarded as pioneers in the metabolic channel to ArsB and ArsC75. ArsB acts as an antiporter that excludes As(III) from cells during the exchange by H+/As(OH)3 attached to the electrochemically mediated proton gradient76. ArsC is the substrate of ArsB that acts as an As(V) reductase. ArsC transforms As(V) to As(III) and increases the array of resistance to include As(V)77.
The biotransformation process of As(V) to As(III) takes place via aquaporin nodulin 26-like intrinsic protein (NIPs)72, which is a water channel that transports water molecules from extracellular to intracellular portions. This reduction reaction occurs in the presence of several reductases that act as electron donors, such as glutaredoxin, glutathione, or thioredoxin78. As an electron donor, glutathione reduces As(V) to As(III) in aqueous solution and forms a complex of arsenotriglutathione, As(III) (GS)3 that immediately gives As(III) to target species that contain dithiol groups79. Methylation mechanisms by the As(III) (GS)3 complex has been discussed in the study of Hayakawa et al.80.
Phytochelatins (PCs), with metal binding capacities, are intracellular thiol-based polypeptides of microalgae. These metalloproteins play a significant role in microalgal detoxification mechanisms. PCs encourage As to bind with thiol groups, i.e. glutathione has a significant function in As complexation and detoxification16. It has been suggested that even at lower As concentrations, the rate of PC synthesis is sufficient to bond with As and cells of marine microalgae species81. The reduction mechanism of As(V) to As(III) is a rapid and important process for further sequestration into the vacuoles of algal cells82 where the majority of As(V) content is reduced within 1–2 days prior to sequestration into the vacuole71,73.
In the present study, the As(III) concentration gradually decreased owing to continuous abiotic oxidation indicating that As(V) constrained the intracellular As(III) accumulation in algal cells69 (Fig. 11). Further, methylation of As(III) to methylated species (MMAA and DMAA) occurred within the cells and their overall concentration gradually increased in the growth medium by their excretion from the cell. However, there are several steps associated with the biochemical metabolism of inorganic As to methylated arsenicals, i.e. mono-di and tri-methyl arsenic species. Some steps are activated by chemical reactions, whereas others are incorporated into enzymatic catalysis. Previously it was found that one catalyst, arsenic-methyltransferase (AS3MT), actively promoted the biotransformation of iAs to methylated arsenic species82. However, the methylation mechanism is an oxidative process where a methyl group (CH3+) is available for the reaction to progress. But, oxidative stress occurred whenever active oxygen come in contact during the catalytic reaction with methyltransferase83. During deviations in the redox state of arsenic, S-adenosylmethionine (SAM) donated methyl groups to As using AS3MT. For methylation mechanisms, SAM is required because the extent and pattern of methylation mechanisms largely depends on its availability84.
Conclusion
The potential growth and As biotransformation by six marine diatom species was investigated under various temperature (0–35 °C) and salinity (0.3–50‰) conditions during three weeks of culture. Except for T. nitzschioides and Skeletonema sp., none of the species biotransformed As species at ≤5 °C and ≥35 °C. However, growth and As biotransformation and subsequent methylation were optimum between temperatures of 10 to 25 °C and salinities of 10 to 35‰. At low salinity levels (0.3–3.5‰), only As(V) was measured in the culture medium, indicating that at such low salinity conditions all the species were unable to reduce As(V) to As(III) or methylated arsenicals. The biological reduction, i.e. biotransformation of As(V) to As(III) and subsequent methylated arsenicals, was significantly different between day 10 and 17 speciation at different temperature and salinity conditions. The interrelated influence of temperature, salinity, and cell size on As biotransformation was also reported for the first time. These results suggest that each species has an optimum temperature and salinity tolerance range suitable for their adaptation metabolism, such as growth and the biotransformation of toxic As(V) to As(III) and further methylation to form methylated As species.
Materials and Methods
Marine diatom species
Six strains of marine diatom species, A. karianus, T. nitzschioides, N. longissima, Skeletonema sp., D. brightwellii, and C. didymus, were used in this study (Fig. 1). Diatom species were supplied by Dr. Kanako Naito, Associate Professor of Hiroshima Prefecture University, Japan. Asteroplanus karianus is a pennate diatom distributed in coastal waters globally, but there is limited information available on its growth, physiology, and life cycle. This strain was isolated in June 2014 from Tsugaru Strait, Hokkaido, Japan. Thalassionema nitzschioides is a yellow-brown pennate diatom with a wide range of salinity tolerance (12–38‰). Nitzschia longissima is a free-living single-celled organism, which is motile and attached to the soft substratum of marine macrophytes, especially on seagrass leaves. Skeletonema sp. is a centric, cylindrical diatom that can survive in water temperatures up to 30 °C and causes water discolouration. Thalassionema nitzschioides, N. longissimi and Skeletonema sp. strains were isolated in June 2015 from Nanaehama, Hokkaido, Japan. Ditylum brightwellii is a marine centric unicellular photosynthetic autotroph. This strain was isolated in March 1989 from Hiroshima Bay, Japan85. Chaetoceros didymus is a photosynthetic centric diatom that is connected in straight chains. This strain was isolated in June 2015 from Nanaehama, Hokkaido, Japan. The cell size and its effect on the biotransformation potentials were taken into consideration during the selection of these six species.
Reagents
Deionized water (arium pro UV, Sartorius Stedim Biotech, Goettingen, Germany) with a resistivity of 18.2 MΩ was used for all experiments. Reagents were commercially available and used without further purification. Sodium hydroxide (NaOH; special grade, Nacalai Tesque, Kyoto, Japan) and hydrochloric acid (HCl; Kanto Chemical, Tokyo, Japan) were used for the pH adjustment of reagents and medium. 4-(2-hydroxyethyl)-1-piperazinyl ethane sulphonate (HEPES; Nacalai Tesque) was used as a buffer reagent in culture medium. Special grade sodium dihydrogen phosphate (NaH2PO4) and disodium hydrogen arsenate heptahydrate (As(V)), both from Wako Pure Chemical (Osaka, Japan), were used as the phosphate and arsenic sources, respectively, in the culture medium.
Preculture and maintenance of marine microalgae
Marine diatom species were maintained in f/2 based nutrient medium (Table 1) in natural sea water. Culture medium and the apparatus (tips, bottles, vessels, micropipettes) were sterilized separately at 121 °C for 30 min in an autoclave (MLS 3780, Sanyo Electric, Japan), followed by UV irradiation for 20 min on a clean bench (NK Clean Bench, VSF-1300A, Nippon, Japan). Before using the diatom species in experiments, cultures were maintained in the same medium for 1–2 weeks in polycarbonate bottles (Nalgene, Nunc; Rochester, NY) until they reached an exponential growth phase in a temperature- and light-controlled incubator (Koitotron3HN-35MLA, Koito Industries, Japan).
Table 1.
Chemical composition of modified f/2 culture medium in artificial seawater.
| Chemicals | Concentration (M) |
|---|---|
| NaNO3 | 8.82 × 10−4 |
| NaH2PO4·2H2O | 3.84 × 10−5 |
| Na2SiO3·9H2O | 3.46 × 10−5 |
| Na2SeO3 | 1.00 × 10−7 |
| CoSO4·7H2O | 4.27 × 10−8 |
| ZnSO4·7H2O | 7.31 × 10−8 |
| MnCl2·4H2O | 9.10 × 10−7 |
| CuSO4·5H2O | 2.80 × 10−8 |
| Na2MoO4·2H2O | 2.89 × 10−8 |
| Vitamin B12 | 3.69 × 10−10 |
| Thiamine HCl | 2.96 × 10−7 |
| Biotin | 2.05 × 10−9 |
Growth and As speciation under various temperature and salinity conditions
The growth and As speciation of diatom species were observed at different temperatures and salinities. For the temperature treatment, diatom cultures were placed into polycarbonate vessels with 30 mL of sterilized culture medium containing natural sea water. The vessels for each batch of culture were kept in an incubator and set at a certain temperature (0, 5, 10, 15, 20, 25, 30, and 35 °C). Artificial sea water (Table S2, Appendix A: Supporting information) at various salinities (0.3, 1.0, 3.5, 5.0, 10, 15, 20, 25, 30, 35, 40, 45, and 50‰) was used to culture the diatom species for the salinity treatment. After incubating the diatom species, As(V) (0.1 µmol L−1) and PO43− (1 µmol L−1) were added to the culture medium. The cultures were grown for three weeks. The liquid samples were collected at day 4, 7, 10, 14, 17, and 21, and filtered using 0.45 µm cellulose membrane filters (Toyo Roshi Kaisha, Tokyo, Japan). Growth of each species was measured spectrophotometrically using a UV-VIS spectrophotometer at 540 nm and was calculated with an established cell density-to-absorbance ratio to estimate cell number. Cell numbers were counted using a digital microscope (Keyence, VHX-1000, Japan). The initial cell concentration of the diatom species was measured as 2.4 × 103 cell mL−1. Growth rate per day was calculated using Eq. (1) 64:
| 1 |
where, N1 = final cell density, N2 = initial cell density, and t = time (day).
Arsenic speciation analysis
Arsenic species in culture media samples were determined using a hydride generation technique according to Hasegawa et al.86. A flame atomic absorption spectrophotometer (AAS) combined with hydride generation device followed by cold trapping (AAS, 170-50A, Hitachi, Japan) was used. Inorganic As (As(V) + As(III)), MMAA(V), DMAA(V) were analysed by adding 5.0 mL 0.20 mol L−1 EDTA·4Na (ethylenediaminetetraacetic acid; Kanto Chemicals) and 5.0 mol L−1 HCl to 40 mL of the sample solution. For As(III), 5.0 mL 0.20 mol L−1 EDTA·4Na and 0.5 mol L−1 potassium hydrogen phthalate (Kanto Chemicals) were added to 40 mL of sample solution. Arsenic species were recorded as a chromatogram on a data processing device (Chromato-PRO, Runtime Instruments, Tokyo, Japan) and the concentration was determined by the peak height. The lowest detectable concentrations of As(III), As(V), MMMA(V), and DMAA(V) were 0.02, 0.11, 0.18, and 0.12 nmol L−1 with RSD values (n = 3) of 1.3, 2.7, 2.5, and 2.3%, respectively87.
Cell size
A minimum of 35 individual cells was measured in each sample using a microscope (KEYENCE, VHX-1000, Japan) at 500X magnification. Cell diameter and height were measured as they appeared on the micro slide glass, considering the cells as cylinders. Cell surface and volume were then calculated in each temperature and salinity condition.
Statistics
Statistical analysis was carried out using SPSS 22.0 for Windows (IBM Co., USA) and Graph Pad Prism 7.0 (GraphPad Software Inc., USA). One-way and two-way analysis of variance (ANOVA) with a Tukey test was conducted to determine significant differences between the means for growth and As biotransformation of each diatom species at each temperature and salinity.
Supplementary information
Acknowledgements
The research has partially been supported by Grants-in-Aid for Scientific Research (15H05118 and 18H03399) from the Japan Society for the Promotion of Science. The authors would like to thank English-speaking editors at Editage (www.editage.jp) for language editing of the manuscript.
Author Contributions
R.I.P. conducted the experiment, wrote the manuscript and drew the figures. K.I. and S.M. helped in measurement of elements and A.A.M. helped in culture experiment. K.N. isolated and maintained the diatom strains. A.S.M., T.M. and H.H. discussed the results. H.H. supervised the study. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rimana Islam Papry, Email: papry015@gmail.com.
Hiroshi Hasegawa, Email: hhiroshi@se.kanazawa-u.ac.jp.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46551-8.
References
- 1.Bahar MM, Megharaj M, Naidu R. Toxicity, transformation and accumulation of inorganic arsenic species in a microalga Scenedesmus sp. isolated from soil. J. Appl. Phycol. 2012;25:913–917. doi: 10.1007/s10811-012-9923-0. [DOI] [Google Scholar]
- 2.Karadjova IB, Slaveykova VI, Tsalev DL. The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat. Toxicol. 2008;87:264–271. doi: 10.1016/j.aquatox.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Niegel C, Matysik F-M. Analytical methods for the determination of arsenosugars—A review of recent trends and developments. Anal. Chim. Acta. 2010;657:83–99. doi: 10.1016/j.aca.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Meharg AA, Hartley-Whitaker J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002;154:29–43. doi: 10.1046/j.1469-8137.2002.00363.x. [DOI] [Google Scholar]
- 5.Wang Y, et al. Review of arsenic speciation, toxicity and metabolism in microalgae. Rev. Environ. Sci. Bio. Technol. 2015;14:427–451. doi: 10.1007/s11157-015-9371-9. [DOI] [Google Scholar]
- 6.Francesconi KA, Edmonds JS. Arsenic and Marine Organisms. Adv. Inorg. Chem. 1996;44:147–189. doi: 10.1016/s0898-8838(08)60130-0. [DOI] [Google Scholar]
- 7.Rahman MA, Hasegawa H, Lim RP. Bioaccumulation, biotransformation and trophic transfer of arsenic in the aquatic food chain. Environ. Res. 2012;116:118–135. doi: 10.1016/j.envres.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Maeda S, Nakashima S, Takeshita T, Higashi S. Bioaccumulation of arsenic by freshwater algae and the application to the removal of inorganic arsenic from an aqueous phase. Part II. by Chlorella vulgaris isolated from arsenic-polluted environment. Sep. Sci. Technol. 2006;20:153–161. doi: 10.1080/01496398508058356. [DOI] [Google Scholar]
- 9.Sanders JG, Osman RW, Riedel GF. Pathways of arsenic uptake and incorporation in estuarine phytoplankton and the filter-feeding invertebrates Eurytemora affinis, Balanus improvisus and Crassostrea virginica. Mar. Biol. 1989;103:319–325. doi: 10.1007/BF00397265. [DOI] [Google Scholar]
- 10.Andreae MO. Distribution and speciation of arsenic in natural waters and some marine algae. Deep-Sea Res. 1978;25:391–402. doi: 10.1016/0146-6291(78)90565-9. [DOI] [Google Scholar]
- 11.Edmonds, J. S. & Francesconi, K. A. In Metal Metabolism in Aquatic Environments (eds William J., Langston & Maria João, Bebianno) 159–183 (Springer, 1998).
- 12.Hasegawa H, et al. Biosynthesis and release of methylarsenic compounds during the growth of freshwater algae. Chemosphere. 2001;43:265–272. doi: 10.1016/S0045-6535(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 13.Gong Y, et al. Effects of arsenate on the growth and microcystin production of Microcystis aeruginosa isolated from Taiwan as influenced by extracellular phosphate. J. Appl. Phycol. 2008;21:225–231. doi: 10.1007/s10811-008-9353-1. [DOI] [Google Scholar]
- 14.Maeda S, Kusadome K, Arima H, Ohki A, Naka K. Biomethylation of arsenic and its excretion by the alga Chlorella vulgaris. Appl. Organomet. Chem. 1992;6:407–413. doi: 10.1002/aoc.590060416. [DOI] [Google Scholar]
- 15.Murray LA, Raab A, Marr IL, Feldmann JR. Biotransformation of arsenate to arsenosugars byChlorella vulgaris. Appl. Organomet. Chem. 2003;17:669–674. doi: 10.1002/aoc.498. [DOI] [Google Scholar]
- 16.Pawlik-Skowronska B, Pirszel J, Kalinowska R, Skowronski T. Arsenic availability, toxicity and direct role of GSH and phytochelatins in As detoxification in the green alga Stichococcus bacillaris. Aquat. Toxicol. 2004;70:201–212. doi: 10.1016/j.aquatox.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Bottino NR, et al. The effects of arsenate and arsenite on the growth and morphology of the marine unicellular algae Tetragelmis Chui (Chlorophyta) and Hymenomonas carterae (Chrysophyta) J. Exp. Mar. Biol. Ecol. 1978;33:153–168. doi: 10.1016/0022-0981(78)90005-9. [DOI] [Google Scholar]
- 18.Fujimoto N, Inamori Y, Sugiura N, Sudo R. Effects of temperature change on algal growth. Environ. Technol. 1994;15:497–500. doi: 10.1080/09593339409385455. [DOI] [Google Scholar]
- 19.Raven JA, Geider RJ. Temperature and algal growth. New Phytol. 1988;110:441–461. doi: 10.1111/j.1469-8137.1988.tb00282.x. [DOI] [Google Scholar]
- 20.Bartley ML, Boeing WJ, Dungan BN, Holguin FO, Schaub T. pH effects on growth and lipid accumulation of the biofuel microalgae Nannochloropsis salina and invading organisms. J. Appl. Phycol. 2013;26:1431–1437. doi: 10.1007/s10811-013-0177-2. [DOI] [Google Scholar]
- 21.Abubakar AL. Effect of salinity on the growth parameters of halotolerant microalgae Dunaliella spp. Nig. J. Basic Appl. Sci. 2017;24:85. doi: 10.4314/njbas.v24i2.12. [DOI] [Google Scholar]
- 22.Foster S, Thomson D, Maher W. Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar. Chem. 2008;108:172–183. doi: 10.1016/j.marchem.2007.11.005. [DOI] [Google Scholar]
- 23.Hemaiswarya S, Raja R, Ravi Kumar R, Ganesan V, Anbazhagan C. Microalgae: a sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2010;27:1737–1746. doi: 10.1007/s11274-010-0632-z. [DOI] [Google Scholar]
- 24.Montagnes DJS, Franklin DJ. Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnol. Oceanogr. 2001;46:2008–2018. doi: 10.4319/lo.2001.46.8.2008. [DOI] [Google Scholar]
- 25.Renaud SM, Thinh L-V, Lambrinidis G, Parry DL. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture. 2002;211:195–214. doi: 10.1016/S0044-8486(01)00875-4. [DOI] [Google Scholar]
- 26.Rao AR, Dayananda C, Sarada R, Shamala TR, Ravishankar GA. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour Technol. 2007;98:560–564. doi: 10.1016/j.biortech.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Saros JE, Fritz SC. Changes in the growth rates of saline-lake diatoms in response to variation in salinity, brine type and nitrogen form. J. Plankton Res. 2000;22:1071–1083. doi: 10.1093/plankt/22.6.1071. [DOI] [Google Scholar]
- 28.Mclachlan J. The effect of salinity on growth and chlorophyll content in representative classes of unicellular marine algae. Can. J. Microbiol. 1961;7:399–406. doi: 10.1139/m61-048. [DOI] [Google Scholar]
- 29.Aydın Gi, Kocata A, Büyükıık B. Effects of light and temperature on the growth rate of potentially harmful marine diatom: Thalassiosira allenii Takano (Bacillariophyceae) Afr. J. Biotechnol. 2009;8:4983–4990. [Google Scholar]
- 30.Berges JA, Varela DE, Harrison PJ. Effects of temperature on growth rate, cell composition and nitrogen metabolism in the marine diatom Thalassiosira pseudonana (Bacillariophyceae) Mar. Ecol. Prog. Ser. 2002;225:139–146. doi: 10.3354/meps225139. [DOI] [Google Scholar]
- 31.Fiala M, Oriol L. Light-temperature interactions on the growth of Antarctic diatoms. Polar Biol. 1990;10:629–636. doi: 10.1007/BF00239374. [DOI] [Google Scholar]
- 32.Javaheri N, et al. Temperature affects the silicate morphology in a diatom. Sci Rep. 2015;5:11652. doi: 10.1038/srep11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia N, et al. Effect of salinity on growth and chemical composition of the diatom Thalassiosira weissflogii at three culture phases. Lat. Am. J. Aquat. Res. 2012;40:435–440. doi: 10.3856/vol40-issue2-fulltext-18. [DOI] [Google Scholar]
- 34.Balzano S, Sarno D, Kooistra WHCF. Effects of salinity on the growth rate and morphology of ten Skeletonema strains. J. Plankton Res. 2010;33:937–945. doi: 10.1093/plankt/fbq150. [DOI] [Google Scholar]
- 35.Aizdaicher NA, Markina ZV. The effect of decrease in salinity on the dynamics of abundance and the cell size of Corethron hystrix (Bacillariophyta) in laboratory culture. Ocean Sci. J. 2010;45:1–5. doi: 10.1007/s12601-010-0001-8. [DOI] [Google Scholar]
- 36.Adenan NS, Yusoff FM, Shariff M. Effect of salinity and temperature on the growth of diatoms and green algae. J. Fish. Aquat. Sci. 2013;8:397–404. doi: 10.3923/jfas.2013.397.404. [DOI] [Google Scholar]
- 37.Salarzadeh A, Ebrahimi E. The effect of temperature and salinity on the growth of Skeletonema costatum and Chlorella capsulata in vitro. Int. J. Life Sci. 2016;10:40–44. doi: 10.3126/ijls.v10i1.14508. [DOI] [Google Scholar]
- 38.Terlizzi DE, Karlander EP. Growth of a coccoid nanoplankter (Eustigmatophyceae) from the Chesapeake Bay as influenced by light, temperature, salinity, and nitrogen source in factorial combination. J. Phycol. 1980;16:364–368. doi: 10.1111/j.1529-8817.1980.tb03046.x. [DOI] [Google Scholar]
- 39.Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensification. 2009;48:1146–1151. doi: 10.1016/j.cep.2009.03.006. [DOI] [Google Scholar]
- 40.Sanchez JF, et al. Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008;79:719–729. doi: 10.1007/s00253-008-1494-2. [DOI] [PubMed] [Google Scholar]
- 41.Martinez ME, Jimnez JM, Yousfi FE. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresource Technol. 1999;67:233–240. doi: 10.1016/S0960-8524(98)00120-5. [DOI] [Google Scholar]
- 42.Christov C, et al. Influence of temperature and methyl jasmonate on Scenedesmus incrassulatus. Biol. Plantarum. 2001;44:367–371. doi: 10.1023/A:1012490610127. [DOI] [Google Scholar]
- 43.Liska AJ, Shevchenko A, Pick U, Katz A. Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol. 2004;136:2806–2817. doi: 10.1104/pp.104.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang W-w, Dong B-z, Cai Z-p, Duan S-s. Growth effects on mixed culture of Dunaliella salina and Phaeodactylum tricornutum under different inoculation densities and nitrogen concentrations. Afr. J. Biotechnol. 2011;10:13164–13174. doi: 10.5897/AJB10.2231. [DOI] [Google Scholar]
- 45.Takagi M, Karseno, Yoshida T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng. 2006;101:223–226. doi: 10.1263/jbb.101.223. [DOI] [PubMed] [Google Scholar]
- 46.Suhendrayatna, Ohki A, Maeda S. Biotransformation of arsenite in freshwater food-chain models. Appl. Organomet. Chem. 2001;15:277–284. doi: 10.1002/aoc.139. [DOI] [Google Scholar]
- 47.Baker J, Wallschlager D. The role of phosphorus in the metabolism of arsenate by a freshwater green alga, Chlorella vulgaris. J. Environ. Sci. (China) 2016;49:169–178. doi: 10.1016/j.jes.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Rahman MA, Hasegawa H. Arsenic in freshwater systems: Influence of eutrophication on occurrence, distribution, speciation, and bioaccumulation. Appl. Geochem. 2012;27:304–314. doi: 10.1016/j.apgeochem.2011.09.020. [DOI] [Google Scholar]
- 49.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/S0378-4274(02)00084-X. [DOI] [PubMed] [Google Scholar]
- 50.Ye J, Rensing C, Rosen BP, Zhu YG. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012;17:155–162. doi: 10.1016/j.tplants.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Luo Z, Yan C. Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa. Environ. Sci. Pollut. Res. Int. 2013;20:7286–7295. doi: 10.1007/s11356-013-1741-7. [DOI] [PubMed] [Google Scholar]
- 52.Knauer K, Hemond H. Accumulation and reduction of arsenate by the freshwater green alga Chlorella sp. (chlorophyta) J. Phycol. 2000;36:506–509. doi: 10.1046/j.1529-8817.2000.99056.x. [DOI] [PubMed] [Google Scholar]
- 53.Duncan, E., Foster, S. & Maher, W. Uptake and metabolism of arsenate, methylarsonate and arsenobetaine by axenic cultures of the phytoplankton Dunaliella tertiolecta. Botanica. Marina. 53, 10.1515/bot.2010.043 (2010).
- 54.Cho SH, et al. Optimum temperature and salinity conditions for growth of green algae Chlorella ellipsoidea and Nannochloris oculata. Fisheries Sci. 2007;73:1050–1056. doi: 10.1111/j.1444-2906.2007.01435.x. [DOI] [Google Scholar]
- 55.Hu H, Gao K. Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol. Lett. 2006;28:987–992. doi: 10.1007/s10529-006-9026-6. [DOI] [PubMed] [Google Scholar]
- 56.Rijstenbil JW. Selection of phytoplankton species in culture by gradual salinity changes. Netherlands J. Sea Res. 1988;22:291–300. doi: 10.1016/0077-7579(88)90031-2. [DOI] [Google Scholar]
- 57.Bergesch M, Garcia M, Odebrecht C. Diversity and morphology of Skeletonema species in Southern Brazil, Southwestern Atlantic Ocean(1) J. Phycol. 2009;45:1348–1352. doi: 10.1111/j.1529-8817.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 58.Kirst GO. Salinity tolerance of eukaryotic marine algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990;41:21–53. doi: 10.1146/annurev.pp.41.060190.000321. [DOI] [Google Scholar]
- 59.Mateos LM, Ordóñez E, Letek M, Gil JA. Corynebacterium glutamicum as a model bacterium for the bioremediation of arsenic. Int. Microbiol. 2006;9:207–215. [PubMed] [Google Scholar]
- 60.Hellweger F, Lall U. Modeling the effect of algal dynamics on arsenic speciation in lake Biwa. Environ. Sci. Technol. 2004;38:6716–6723. doi: 10.1021/es049660k. [DOI] [PubMed] [Google Scholar]
- 61.Wang NX, et al. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res. 2013;47:2497–2506. doi: 10.1016/j.watres.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 62.Duncan EG, Maher WA, Foster SD. Contribution of arsenic species in unicellular algae to the cycling of arsenic in marine ecosystems. Environ. Sci. Technol. 2015;49:33–50. doi: 10.1021/es504074z. [DOI] [PubMed] [Google Scholar]
- 63.Latala A, Hamoud N, Plinski M. Growth dynamicsand morphology of plankton green algae from brackishwaters under the influence of salinity, temperature andlight. Acta Ichthyologica Et Piscatoria. 1991;21:101–116. doi: 10.3750/AIP1991.21.S.11. [DOI] [Google Scholar]
- 64.Abu-Rezq TS, Al-Musallam L, Al-Shimmari J, Dias P. Optimum production conditions for different high-quality marine algae. Hydrobiologia. 1999;403:97–107. doi: 10.1023/A:1003725626504. [DOI] [Google Scholar]
- 65.Hirata H, Andarias I, Yamasaki S. Effects of salinity and temperature on the growth of the marine phytoplankton Chlorella saccharophila. Mem. Fac. Fish., Kagoshima Univ. 1981;30:257–262. [Google Scholar]
- 66.S. TR, Ayala-Silva T, B. Dunn C, G. GG. Effects of arsenic on nutrient accumulation and distribution in selected ornamental Plants. Agric. Sci. 2015;06:1513–1531. doi: 10.4236/as.2015.612145. [DOI] [Google Scholar]
- 67.Guo P, Gong Y, Wang C, Liu X, Liu J. Arsenic speciation and effect of arsenate inhibition in a Microcystis aeruginosa culture medium under different phosphate regimes. Environ. Toxicol. Chem. 2011;30:1754–1759. doi: 10.1002/etc.567. [DOI] [PubMed] [Google Scholar]
- 68.Ullrich-Eberius CI, Sanz A, Novacky AJ. Evaluation of arsenate- and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J. Exp. Bot. 1989;40:119–128. doi: 10.1093/jxb/40.1.119. [DOI] [Google Scholar]
- 69.Hellweger FL, Farley KJ, Lall U, Di Toro DM. Greedy algae reduce arsenate. Limnol. Oceanogr. 2003;48:2275–2288. doi: 10.4319/lo.2003.48.6.2275. [DOI] [Google Scholar]
- 70.Rahman MA, Hassler C. Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquat. Toxicol. 2014;146:212–219. doi: 10.1016/j.aquatox.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Levy JL, et al. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum) Environ. Toxicol. Chem. 2005;24:2630–2639. doi: 10.1897/04-580R.1. [DOI] [PubMed] [Google Scholar]
- 72.Zhao FJ, Ma JF, Meharg AA, McGrath SP. Arsenic uptake and metabolism in plants. New Phytol. 2009;181:777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- 73.Cullen WR, Li H, Pergantis SA, Eigendorf GK, Harrison LG. The methylation of arsenate by a marine alga Polyphysa peniculus in the presence of 1-methionine-methyl-d3. Chemosphere. 1994;28:1009–1019. doi: 10.1016/0045-6535(94)90017-5. [DOI] [Google Scholar]
- 74.Yang HC, Fu HL, Lin YF, Rosen BP. Pathways of arsenic uptake and efflux. Curr Top Membr. 2012;69:325–358. doi: 10.1016/B978-0-12-394390-3.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hansen HR, Raab A, Francesconi KA, Feldmann I. Metabolism of arsenic by sheep chronically exposed to arsenosugars as a normal part of their diet. 1. Quantitative intake, uptake, and excretion. Environ. Sci. Technol. 2003;37:845–851. doi: 10.1021/es026074n. [DOI] [PubMed] [Google Scholar]
- 76.Meng YL, Liu Z, Rosen BP. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli. J. Biol. Chem. 2004;279:18334–18341. doi: 10.1074/jbc.M400037200. [DOI] [PubMed] [Google Scholar]
- 77.Gladysheva TB, Oden KL, Rosen BP. Properties of the arsenate reductase of plasmid R773. Biochemistry. 1994;33:7288–7293. doi: 10.1021/bi00189a033. [DOI] [PubMed] [Google Scholar]
- 78.Yin X, Wang L, Duan G, Sun G. Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii. J. Environ. Sci. 2011;23:1186–1193. doi: 10.1016/s1001-0742(10)60492-5. [DOI] [PubMed] [Google Scholar]
- 79.Scott N, Hatlelid KM, MacKenzie NE, Carter DE. Reactions of arsenic (III) and arsenic (V) species with glutathione. Chem. Res. Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- 80.Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005;79:183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- 81.Morelli E, Mascherpa MC, Scarano G. Biosynthesis of phytochelatins and arsenic accumulation in the marine microalga Phaeodactylum tricornutum in response to arsenate exposure. Biometals. 2005;18:587–593. doi: 10.1007/s10534-005-2998-1. [DOI] [PubMed] [Google Scholar]
- 82.Zhang SY, Sun GX, Yin XX, Rensing C, Zhu YG. Biomethylation and volatilization of arsenic by the marine microalgae Ostreococcus tauri. Chemosphere. 2013;93:47–53. doi: 10.1016/j.chemosphere.2013.04.063. [DOI] [PubMed] [Google Scholar]
- 83.Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NF- B DNA binding activity and related gene expression. Toxicol. Lett. 2002;133:33–45. doi: 10.1016/S0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 84.Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- 85.Yamaguchi M. Physiological ecology of the red tide flagellate Gymnodinium nagasakiense (Dinophyceae)—mechanism of the red tide occurrence and its prediction. Bull. Nansei Natl. Fish. Res. Inst. 1994;27:251–394. [Google Scholar]
- 86.Hasegawa H, Sohrin Y, Matsui M, Hojo M, Kawashima M. Speciation of arsenic in natural waters by solvent extraction and hydride generation atomic absorption spectrometry. Anal. Chem. 1994;66:3247–3252. doi: 10.1021/ac00091a039. [DOI] [Google Scholar]
- 87.Mamun MAA, et al. Comparative biotransformation and detoxification potential of arsenic by three macroalgae species in seawater: Evidence from laboratory culture studies. Chemosphere. 2019;228:117–127. doi: 10.1016/j.chemosphere.2019.04.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.