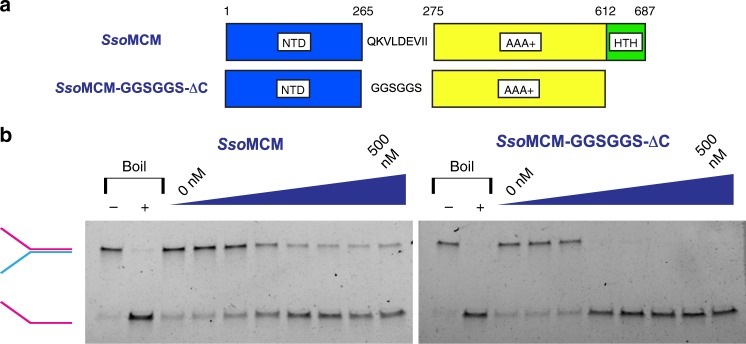
Fig. 1.
The MCM construct of this study, SsoMCM-GGSGGS-ΔC, unwinds DNA. a The fundamental domain organization of MCM proteins includes an N-terminal domain (NTD), an AAA+ ATPase domain, and a C-terminal helix-turn-helix domain (HTH). The MCM construct of the current structural study consists of SsoMCM with the 9-residue (QKVLDEVII) linker between the NTD and the AAA+ domain shortened to six residues and also mutated to the sequence GGSGGS. The construct also uses a previously described deletion of the HTH domain at residue 612 (ref. 31). b The SsoMCM-GGSGGS-ΔC construct displaces a labeled strand from a fork DNA substrate at least as well as wild-type full-length SsoMCM. Protein concentrations were titrated from 0 to 500 nM (monomer), and reactions were incubated at 69 °C for 60 min. Reactions consisted of 25 mM HEPES (pH = 7.6), 10 mM NaCl, 5 mM Mg(OAc)2, 4 mM ATP, and 3.7 nM fluorescein-labeled DNA substrate