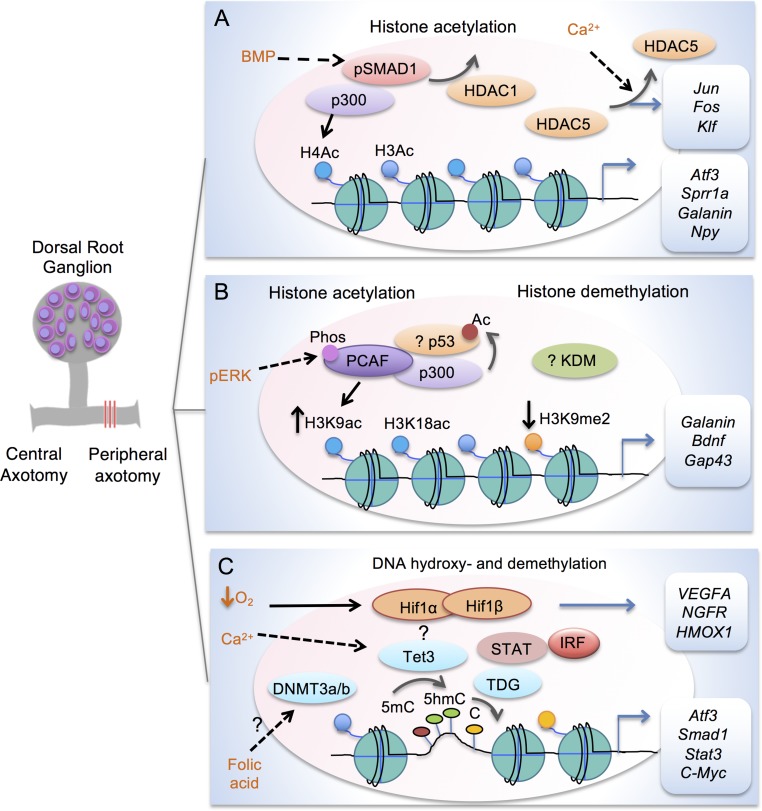
Figure 2.
Transcription modules in regulating axon growth potential of DRG neurons after peripheral axotomy. (A) Schematic depiction of transcriptional module comprised of Smad1 and p300 to enhance histone acetylation in conditioned DRG at target loci (Atf3, Sprr1a, Galanin and Npy). Promoter occupancy of Smad1 helps recruitment of p300 and displacement of HDAC1. Peripheral axotomy also triggers nuclear exit of HDAC5 in response to retropropagation of calcium wave, leading to induction of RAGs (Jun, Fos, and Klf). (B) Transcriptional module comprised of p53 and p300/PCAF regulates H3K9ac and H3K18ac at target RAGs (Galanin, Bdnf, Gap43 and Coronin1b) in conditioned DRG. Acetylation of p53 is also increased by HATs in cortical neurons. Retrograde signaling of pERK results in threonine phosphorylation and nuclear localization of PCAF. In conditioned DRG, H3K9me2 is decreased regulated by a yet unknown KDM. (C) Epigenetic factors in mediating DNA hydroxy- and demethylation in conditioned DRG. Peripheral axotomy triggers Tet3 upregulation, which may be dependent on Calcium wave. Tet3 catalyzes conversion of 5mC to 5hmC, while TDG mediates conversion back to C, resulting in RAG induction of Atf3, Smad1, Stat3, and C-Myc. Folate may influence DNA methylation through DNMT3a/3b. Tet3 may partner with HIF, STAT, and IRF for 5hmC reconfigurations. Hypoxia stabilizes HIF complex to induce target genes, e.g. VEGFA, NFGR and HMOX1.