Figure 6.
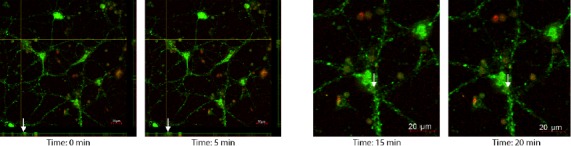
Live imaging of primary cortical neurons undergoing mitochondrial transfer. Rat E18 neuronal cells were harvested and seeded in poly‐D‐lysine coated (100 μg/mL) 6‐well plates at 1.5 × 106 cells/well in Dulbecco's Modified Eagle Media high glucose with 1% antibiotic/antimycotic for 24 h. The media was changed every 3 d, and the cells were subcultured at 90% confluency as needed. Twenty‐four hours prior to the preconditioning, U87 astrocytes were stained with MitoTracker Deep Red FM (500 nmol/L) according to manufacturer's protocol and seeded into coculture inserts at 0.5 × 106 cells/well. On the day of the experiment, rat E18 neuronal cells were stained with MitoTracker Green FM (200 nmol/L) according to manufacturer's protocol. The neuronal cells were then cocultured with U87 astrocytes for 3 h prior to Hyperbaric oxygen therapy (HBOT) administration. The cells were subjected to 70 min of HBOT at 2.5 ATA with 10 min pressurization and depressurization at a rate of 0.07 atm/min for a total of 90 min. Directly following HBOT treatment, the cocultured astrocytes were removed and the confocal z‐stacks live images were captured at 180×. Primary rat neuronal cell (PRNC) mitochondria: Green; Astrocyte mitochondria: Red. White arrows indicate the movement of astrocyte mitochondria into the PRNC during 5‐min intervals. The scale bar corresponds to 20 μm
