Abstract
Bone disease is a serious complication to diabetes. Patients with type 1 diabetes (T1D) and type 2 diabetes (T2D) suffer from an increased risk of fracture, most notably at the hip, compared with patients without diabetes. Confounders such as patient sex, age, body mass index, blood glucose status, fall risk, and diabetes medications may influence the fracture risk. Different underlying mechanisms contribute to bone disease in patients with diabetes. Bone quality is affected by low bone turnover in T1D and T2D, and furthermore, incorporation of advanced glycation end-products, changes in the incretin hormone response, and microvascular complications contribute to impaired bone quality and increased fracture risk. Diagnosis of bone disease in patients with diabetes is a challenge as current methods for fracture prediction such as bone mineral density T-score and fracture risk assessment tools underestimate fracture risk for patients with T1D and T2D. This review focuses on bone disease and fracture risk in patients with diabetes regarding epidemiology, underlying disease mechanisms, and diagnostic methods, and we also provide considerations regarding the management of diabetes patients with bone disease in terms of an intervention threshold and different treatments.
Keywords: Diabetes, Bone quality, Osteoporosis
1. Introduction
Diabetes mellitus type 1 (T1D) and type 2 (T2D) affects millions of people worldwide. Diabetes is characterized by hyperglycemia and diabetes complications that affect quality of life of the patients negatively and places a major economic burden on society [1]. Thus, diagnosis, treatment and prevention of diabetes and its complications is crucial. Traditional diabetes complications are microvascular complications; retinopathy, nephropathy, and neuropathy, and macrovascular complications such as cardiovascular disease. Over the last 50 years, it has become evident that diabetes also affects bone and patients with diabetes have an increased risk of fracture. The diabetic bone disease is caused by complex underlying mechanisms leading to impaired bone quality. Furthermore, an increased risk of falling in diabetes patients and possible harmful side effects secondary to diabetes treatment may further increase the fracture risk. Interestingly, traditional fracture predictors such as the bone mineral density (BMD) [2] and The Fracture Risk Assessment (FRAX) tool [2,3] underestimate fracture risk in diabetes, thus contributing to the notion that bone quality is affected by diabetes. The current knowledge on diabetic bone disease and fracture risk in patients with diabetes regarding epidemiology, underlying disease mechanisms, diagnosis, and treatment, and future perspectives in managing bone disease in diabetes are reviewed in the following.
2. Fracture risk
Fracture risk in patients with diabetes can be described by fracture site, diabetes type, possible confounders, and fracture mechanism (low/high energy). This review focuses on low-energy or fragility fractures.
2.1. Fracture site
Meta-analyses have evaluated fracture risk in patients with diabetes. For T1D, recent studies report an increased relative risk (RR) of hip fracture of 4.5–5.5 compared with controls without diabetes [[4], [5], [6]]. Other studies have reported higher RRs for hip fracture of 6–7 for T1D compared with controls [7,8]. For T2D, most studies report an increased RR of hip fracture of 1.2–1.3 compared with controls without diabetes [4,5,7,8]. In addition to hip fracture, the risk of fractures at other sites may also be increased. For overall fracture risk, one study reported increased RRs of all fractures of 1.51 and 1.22 for T1D and T2D, respectively, compared with controls [4]. Two other studies found similar estimates for overall fracture risk in T2D [9] and also for so-called low bone mass-related fractures in T2D [10]. For vertebral fractures, the published data show insignificant results for both T1D and T2D [4,11]. However, one study comprising patients with both T1D and T2D did find increased RR of vertebral fractures compared with controls [12]. The reported differences in risk estimates for fractures may be due to heterogeneity of the studies included in the meta-analyses. Some meta-analyses include only cohort studies with a prospective or retrospective design, while other meta-analyses are based on all study types including cross-sectional studies. Further, not all original studies have a clear definition of diabetes type [[13], [14], [15]]. If diabetes definition is based on registry data, insulin use, or age at diabetes diagnosis, some patients with T1D could be misclassified as having T2D. This would bias the results and potentially increase the estimates for fracture risk in T2D.
2.2. Diabetes type
Most studies report higher fracture risks for patients with T1D than for patients with T2D compared with controls. T1D and T2D are both characterized by hyperglycemia, but the underlying disease mechanisms are very different [16]. In T1D, autoimmune destruction of the pancreatic beta cells results in insulinopenia, and patients with T1D are hence total reliant on treatment with exogenous insulin. Patients with T1D are often diagnosed in early childhood or adolescence and have no diabetes-related complications at time of diagnosis. In contrast, T2D is triggered by insulin resistance and relative insulin deficiency. Patients with T2D are often older than patients with T1D at the time of diagnosis and may present with a number of diabetes complications already at time of diagnosis. As patients with T1D lack insulin during childhood and adolescence, they fail to reach the expected peak bone mass in young life, thus increasing risk of fracture, as reviewed by Hough et al. [17]. Furthermore, insulin treatment may affect bone turnover, as reviewed later in this review. For T2D, bone turnover may be affected by obesity and hyperglycemia in the years prior to diagnosis and treatment.
2.3. Other confounders
For gender, a number of studies have reported an increased fracture risk in female patients with T1D and T2D compared with male patients with T1D and T2D, respectively [10,18,19]. Other studies reported no effect of gender on fracture risk [5,12,13]. Leslie et al. [14] investigated the effect of age on fracture risk in a population of patients with diabetes. However, there was no clear definition of diabetes type and the cohort comprised patients with both T1D and T2D. The study revealed a biphasic pattern where the risk of osteoporotic fractures and hip fractures is first decreased in the years following diagnosis of diabetes but then subsequently increased in patients with long diabetes duration. The same study showed that the fracture rates for patients with diabetes were higher in younger age compared with controls without diabetes but lower in older age compared with controls, thus emphasizing the complex relationship between fracture risk and age in patients with diabetes [14]. For diabetes duration, studies have shown positive associations with fracture risk [20], and presence of microvascular complications is also associated with increased fracture risk [21,22]. For blood glucose, a positive association has been shown between glycosylated hemoglobin (HbA1c) and fracture risk in T1D [23] and T2D [24], however a recent study showed only increased risk of fracture in patients with T1D and HbA1c above 8%, while HbA1c levels did not affect fracture risk in T2D [25]. Two other studies by Puar et al. [26] and Holmberg et al. [27] reported inverse relationships between blood glucose and fracture risk. Puar et al. [26] showed that HbA1c was inversely correlated to fracture risk in patients with T2D, and the authors proposed that this is due to and increased risk of falling with hypoglycemia. Holmberg et al. [27] conducted a large prospective study and found that hyperglycemia when measured as blood glucose 2 hours after an oral glucose tolerance test was inversely associated with incidence of multiple fractures in men and women, and in women also with osteoporotic fractures. In the study, blood glucose was positively associated with body mass index (BMI), and the authors speculated whether the results reflect a protective effect of obesity on fracture risk in combination with a harmful effect of smoking in the leaner patients [27]. Thus, poor glycemic control may impact differently on fracture risk dependent on diabetes type.
For weight, Huang et al. [28] have previously presented the complex relationship between obesity and fracture risk. The authors showed in a large retrospective study, that obesity was positively associated with T2D. Interestingly, the study also showed that for patients with T2D, high BMI was associated with a lower risk of hip fracture compared with normal BMI [28]. However, when comparing different BMI-subgroups, the RR of hip fracture was only significantly increased for the T2D patients with a normal BMI compared with controls with similar BMI [28]. The study lacks information on BMD, which could potentially confound the results. In a cross-sectional study, both underweight and overweight in T2D were associated with the presence of vertebral fractures [29]. Furthermore, BMI has been proposed as a factor in prediction models for the risk of hip fracture and presence of vertebral fractures in T2D [30,31]. A tentative conclusion on the relationship between weight and fracture is that obesity is a contributing factor to disease development of T2D but may also protect against fragility fractures. Increased frailty in patients with diabetes may also contribute to fracture risk. In a study by Li et al., patients with T2D were more prone to frailty than population controls, and higher frailty increased the risk of fragility fracture but not spine or hip fracture in T2D [32]. Frailty may potentially lead to falls, and a study showed that patients with diabetes reported more falls which was associated with low energy fractures [33]. In addition, sarcopenia, the presence of low muscle mass and low muscle function [34] has been positively associated with osteoporosis, fracture risk, and fall risk in postmenopausal women without diabetes [35]. For patients with T2D, a large cohort study from Korea reported that 15.7% of patients with T2D compared with 6.9% of controls suffered from sarcopenia [36]. However, the study defined sarcopenia as low skeletal muscle mass measured by dual-energy X-ray absorptiometry (DXA) scans and did not investigate muscle function. A cross-sectional study with young patients age 40–60 years found lower muscle mass and decreased muscle function in patients with T2D compared with controls, even after adjustment for disease duration, glucose status, vitamin D levels, and presence of microvascular complications [37]. There is still a lack of studies investigating the association between sarcopenia and fracture risk in patients with diabetes. Taken together, several confounders may influence the risk of fracture in diabetes. The possible confounders of fracture risk should be addressed in studies examining fracture risk in patients with diabetes.
2.4. Morbidity and mortality related to fracture in diabetes
Hip fractures are known to negatively impact morbidity and mortality in all patients [38,39], including patients with diabetes [40]. For proximal humerus fractures, one study showed that women with T2D had increased mortality following fracture and both men and women with T2D had a higher number of postfracture complications compared with patients without diabetes [41]. Interestingly, another study that could not discriminate between T1D and T2D showed that patients with diabetes did not have increased mortality nor longer duration of hospital stay following proximal femur fracture compared with patients without diabetes [42]. Fracture incidence in patients with diabetes influences the quality of life, and a study by Vokó et al. showed that osteoporotic fractures decrease the quality of life to the same extent as severe diabetes complications such as blindness and amputation [43]. Taken together, these data suggest that fractures in patients with diabetes is a serious diabetes complication.
3. Underlying mechanisms
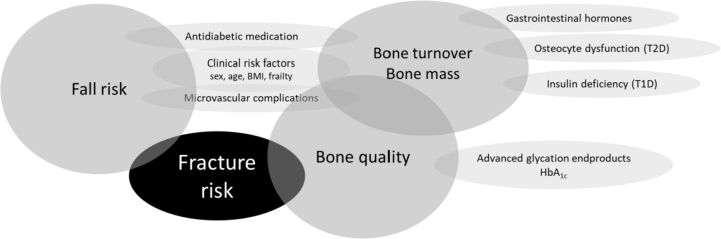
In diabetes, bone disease is caused by underlying mechanisms that impair bone quality and increase fracture risk. Here, we explore bone quality by bone turnover and bone material properties. For an overview of all factors affecting fracture risk in patients with diabetes, see Fig. 1.
Fig. 1.
Summary of the different factors that contribute to the increased fracture risk in patients with diabetes, see text for further details. BMI, body mass index; T1D, type 1 diabetes; T2D, type 2 diabetes; HbA1c, glycosylated hemoglobin.
3.1. Bone turnover
Bone turnover in patients with diabetes is lower compared with controls without diabetes. Bone biopsy studies have shown low bone turnover in T1D and T2D, although interpretation of the results is limited by small numbers of patients in the studies [44,45]. In contrast, another study with transiliac biopsies in 18 patients with T1D and subsequent histmorphometric analyses found no differences in neither bone turnover nor structural parameters between the patients and a matched control group without diabetes, however, patients in this study were recently diagnosed and well-regulated with a mean HbA1c of 6.8% [46]. A meta-analysis from our group have shown lower levels of bone turnover markers in patients with T1D and T2D compared with controls without diabetes [47]. Our study demonstrated lower serum levels of the resorption marker C-terminal cross-linked telopeptide (CTX) and of the formation markers procollagen type 1 amino terminal propeptide (P1NP) and osteocalcin in patients with diabetes as a whole compared with controls without diabetes. This was also found when patients with T1D or T2D were compared separately with controls [47]. Interestingly, the study showed higher serum levels of sclerostin in patients with T1D and T2D compared with controls. Sclerostin, a bone-signaling peptide, is secreted from the osteocyte and inhibits osteoblast activity by blocking the canonical wingless integration signaling and stimulates osteoclast activation by stimulating the release of receptor activator of nuclear factor-κB ligand (RANKL) from osteocytes [48]. Secretion of sclerostin by the osteocytes is reduced by mechanical loading [49], and women with T2D and relative immobility have increased levels of sclerostin [50]. In vitro studies have shown increased sclerostin expression in osteoblasts, osteocytes, and osteocyte-like cells after incubation with glucose at high concentrations [51,52]. A paper by Daniele et al. [53] demonstrated that sclerostin levels are increased in patients with impaired fasting glucose compared with patients with normal fasting glucose and correlated with peripheral insulin resistance. Taken together, sclerostin appears to be linked with hyperglycemia in prediabetes and diabetes, and we speculate whether changes in sclerostin levels in diabetes may be caused by osteocyte dysfunction based on hyperglycemia, relative immobility, and possible additional factors.
3.2. Bone material properties
Advanced glycation end-products (AGEs) are incorporated into bone by nonenzymatic glycation of collagen and may affect the bone material properties. AGEs accumulate in tissues and bone with age. Increased levels of AGEs have been linked with osteoporosis in postmenopausal women [54], and another study has shown that levels of pentosidine, the most common AGE, all AGEs, and the soluble receptor for AGEs, sRAGE, were higher in patients with T2D compared with healthy controls [55]. In vitro studies indicate that AGEs interfere with osteoclast differentiation and activity [56] and also with osteoblast function [57]. Animal studies have shown that AGE-modified collagen inhibited osteoblastic differentiation and function in vitro [58].
3.3. Gastrointestinal hormones
Changes in the gastrointestinal hormone response may also contribute to bone disease in diabetes. The incretin hormones glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) and the gastrointestinal hormone glucagon-like peptide-2 (GLP-2) are secreted from the L- (GLP-1 and GLP-2) and K-cells (GIP) of the small intestine following food intake. GIP and GLP-1 exert a beneficial effect of glucose homeostasis and previous studies have found that T2D patients have a decreased incretin response [[59], [60], [61]] which could contribute to disease development. On the contrary, for T1D, results have been conflicting [[62], [63], [64]]. The gastrointestinal hormones are thought to affect bone turnover through the gut-bone-axis [65]. Receptors for GIP have been found on osteoblast-like cells [66], mature osteoblasts [67], and osteoclasts [68] and act in favor of bone formation. Receptors for GLP-1 and GLP-2 have been found on osteoblastic precursor cells and treatment of the cells with GLP-1 and GLP-2 increased cell viability [67]. For GLP-1, the presence of a functional receptor on mature osteoblasts has been suggested in some papers [67,69] but denied by others [70,71]. Taken together, a lack of proof remains that the gastrointestinal hormones contribute to bone disease in diabetes.
3.4. Microvascular complications
Microvascular complications may affect the bone in diabetes as argued by Shanbhogue et al. [72] in a review from 2017 that outlines the possible associations between microangiopathy, bone loss, and fracture risk in T1D and T2D. Several other papers have reported that peripheral neuropathy [21,73] and retinopathy [74,75] are associated with fracture risk in T2D and possibly also T1D. However, other studies have suggested that microvascular complications do not contribute to increased fracture risk in T1D and T2D [76]. A register-based case-control study with 124,655 fracture cases and 373,962 age- and sex-matched controls showed that only diabetes nephropathy but no other diabetes complication increased the risk of fracture for patients with T1D and T2D [77]. The question remains whether microangiopathy in relation to bone disease in diabetes serves as a proxy for diabetes duration, poorly controlled diabetes, and an increased risk of falling. Some prospective studies with relatively long follow-up and a large number of participants have shown that the association between fractures risk in patients with T2D persists after adjusting for increased fall risk [15,78].
4. Diagnosis of bone disease in diabetes
The bone status and fracture risk in patients with diabetes may be evaluated by different approaches; BMD, clinical risk factors, fracture probability, bone microarchitecture, and bone strength.
4.1. BMD and clinical risk factors
Traditionally, fracture risk is assessed by DXA-derived BMD and T-scores. Studies have consistently shown lower BMD in patients with T1D compared with controls without diabetes [8], and higher BMD in patients with T2D [8,79]. Remarkably, for patients with both T1D and T2D, the BMD T-score underestimates the fracture risk [8,79]. A paper compiling data from three prospective studies found that women and men with T2D had a higher risk of hip and nonspine fracture for a given T-score compared with controls without diabetes, however, for male patients not using insulin, the increased fracture risk was only significant for hip fractures [79]. Further, a large cohort study found that both the femoral neck BMD T-score and the prevalence of major fracture were significantly higher in women with T2D compared with women without diabetes [80]. The same study also found that low BMD was associated with incident fracture risk in patients with T2D similar to the risk in patients without diabetes [80]. This has also been shown in another large cohort study [79]. The FRAX algorithm is a computer-based tool that allows for calculations of the 10-year fracture probability [3]. The assessment is based on clinical risk factors and the hip BMD T-score and allows for incorporation of secondary osteoporosis such as T1D but not T2D. One prospective study found that the FRAX algorithm underestimates fracture risk in patients with T2D [79], and a retrospective cohort study found that FRAX underestimates the risk of hip fracture and major osteoporotic fracture in a mixed group of T1D and T2D patients [81]. In conclusion, neither BMD T-score nor the FRAX tool provide satisfactory fracture risk assessment for patients with diabetes, and further considerations on this topic are described in section 5.1.
4.2. Bone microarchitecture and bone quality
High resolution peripheral quantitative computed tomography (HRpQCT) evaluates bone microarchitecture and volumetric BMD (vBMD) at the tibia and radius and the estimated bone strength and failure load can be calculated. A large cohort study has shown that HRpQCT-derived bone indices including estimated failure load predict fracture risk in patients without diabetes [82]. A newer approach to assess bone quality is bone indentation. Bone indentation can be performed with a hand-hell probe that creates small microcracks on the bone surface in vivo. The bone material strength index (BMSi) is calculated based on the bone’s resistance to indentation [83,84]. Samelson et al. [85] reported lower cortical vBMD and higher cortical porosity in the tibia but not the radius in a prospective cohort study with 129 patients with T2D compared with controls without diabetes. A cross-sectional study of 99 women with T2D found that the cortical porosity was higher in the radius in T2D compared with controls when measured at the standard site of the radius but lower in T2D compared with controls when measured at a more proximal site [86]. There were no differences between groups in cortical porosity of the tibia. The study argues that it may be more accurate to measure cortical indices at a more proximal site due to lower risk of misplacement of the endosteal contour. The same study found lower BMSi and higher failure load in both radius and tibia in the patients with T2D compared with controls [86]. A cross-sectional study with approximately 100 patients with T1D and 100 patients with T2D found that bone microarchitecture when evaluated by HRpQCT in the tibia and radius did not differ between the two groups [87]. Another cross-sectional study with 55 patients with T1D found that at the radius, T1D patients with microvascular diabetes complications had lower vBMD and cortical thickness and larger total and trabecular bone cross-sectional areas compared with the control group. The differences were notsignificant for patients without microvascular complications. There was no differences between the groups where observed concerning the tibia [88]. A cross-sectional study by Farr et al. [89] found no differences in bone microarchitecture including cortical porosity in women with T2D compared with women without diabetes. Interestingly, the same study reported lower BMSi of the tibia in the patients with T2D compared with the controls. Taken together, available data for patients with diabetes points towards deficits in the cortical compartment and a lower resistance to indentation. In combination with a low bone turnover, bone microcracks may occur caused by impaired bone quality, and the fracture risk is increased. Finally, the trabecular bone score (TBS) derived from DXA images may also contribute to assessment of bone quality. Leslie et al. [90] evaluated 2356 women with diabetes (both T1D and T2D) and 27,051 women without diabetes and found lower TBS in patients with diabetes compared with controls despite higher lumbar spine and hip BMD in the patients with diabetes. Furthermore, TBS predicted the fracture risk in both patients with diabetes and controls independently of BMD. Lower TBS values despite higher BMD have also been reported by other studies in patients with T2D [91] and in a group of patients with both T1D and T2D [92].
5. Management of bone disease in diabetes
For the management of bone disease in diabetes, the following should be considered: diagnosis of bone disease, intervention threshold for antiosteoporotic treatment, effectiveness of antiosteoporotic treatment in diabetes, and treatment of hyperglycemia and other diabetes complications.
5.1. Diagnosis and intervention threshold
A review by Ferrari et al. [93] on behalf of the Bone and Diabetes Working Group of the International Osteoporosis Foundation proposed an algorithm for fracture risk evaluation in adult diabetes patients. The authors suggest that incident hip or vertebral fragility fractures indicate start of antiosteoporotic treatment. In cases of fractures at other sites or if no fracture, patients with diabetes should be further evaluated by DXA and clinical risk factors [93]. As mentioned previously, BMD T-score and FRAX-derived fracture probability estimates underestimate fracture risk in patients with T1D and T2D. Previous studies have suggested that the intervention threshold for patients with diabetes should be altered, parallel to clinical guidelines for glucocorticoid-induced osteoporosis where treatment is initiated at a higher BMD T-score than for primary osteoporosis [94]. Schwartz et al. [79] found that for risk of hip fracture, the BMD T-score is 0.59 higher in women and 0.38 higher in men with T2D compared with women and men, respectively, without diabetes. Hence, we propose that for BMD T-score, the intervention threshold for patients with diabetes should be increased to −2.0, as also suggested by Ferrari et al. [93]. For FRAX, T2D should be included as a risk factor for secondary osteoporosis in future versions of the algorithm.
5.2. Antiosteoporotic treatment in diabetes
Data is sparse regarding the effect of antiosteoporotic medication in patients with T1D and T2D, and so far, published papers are either based on post hoc subgroup analyses of randomized trials or from registry-based studies. For alendronate, post hoc analysis of data from a randomized trial showed that the effect of alendronate on BMD gain was not affected by diabetes status in women with T2D [95]. Two registry-based studies found similar effects of alendronate treatment on risk of major osteoporotic fracture in patients with T2D and controls [96], on risk of any fracture for diabetes as a group compared with controls [97], and on risk of hip fracture for T1D and T2D compared with controls, respectively [97]. For risedronate, a post hoc analysis of Japanese phase 3 trials found the same beneficial effect on bone turnover and BMD in patients with T2D compared with controls [98]. There is currently no evidence of the effect of treatment with denosumab on bone status and fracture risk in patients with diabetes. For teriparatide, post hoc analyses of a randomized trial showed that the effects of teriparatide on non-vertebral fracture risk and BMD gain were similar in patients with T2D and controls without diabetes, furthermore the T2D patients had a larger increase in femoral neck BMD during 18 months of treatment with teriparatide compared with controls [99]. A potential new antiosteoporosis treatment; Romozosumab, is a sclerosin antibody thus causing a loss of osteoblast inhibition in combination with inhibition of osteoclast recruitment and activation. Romozosumab has been shown to increase BMD at different skeletal sites and decrease fracture risk compared with placebo or other antiosteoporotic treatments [100]. As increased sclerostin levels in T1D and T2D may contribute to bone disease, it will be very interesting to investigate the effect of Romozosumab in patients with diabetes.
5.3. Treatment of hyperglycemia and diabetes complications
Hyperglycemia should be treated with antidiabetic medication to avoid detrimental effects of diabetes including microvascular complications, while considering possible side effects of the antidiabetic drug on bone. Treatment of T1D and T2D relies mainly on antidiabetic medication, with insulin as the sole treatment of T1D, and a combination of antidiabetic drugs for T2D in addition to lifestyle changes and also bariatric surgery. Numerous studies have reported on the effects of antidiabetic medication on bone turnover and fracture risk, but many studies are retrospective or registry-based, thus limiting the interpretation of the results.
5.4. Traditional oral antidiabetic medication
Metformin is the first line therapy in T2D. Studies have shown osteogenic effects of metformin both in vitro in bone marrow progenitor cells and in vivo in a diabetes rat model, by increasing the expression of osteoblast-specific transcription factor Runx2 [101] and by promoting osteoblastic differentiation in osteoblast-like cells [102]. A 2-year prospective clinical trial reported lower levels of P1NP in a group of T2D patients treated with metformin alone or a combination of metformin and rosiglitazone compared with rosiglitazone alone or different combinations of insulin treatment [103]. For fracture risk, metformin has been associated with possible fracture reduction [76,[104], [105], [106]] or with no effect [107]. Sulphonylureas are widely used in T2D treatment but hypoglycemia is a common side effect to treatment and may increase fracture risk. In an animal study, the sulphonylurea glimepiride was shown to intensify bone formation in ovariectomized rats [108]. For fracture risk, studies have reported different outcomes with sulphonylurea treatment: neutral effect on fracture risk [76,106], increased fracture risk [106,107,109], or decreased fracture risk [104]. Glitazones were previously widely used in the treatment of T2D but their use is now limited due to harmful side effects such as an increased risk of myocardial infarction with rosiglitazone [110], and an increased risk of bladder cancer with pioglitazone [111]. Concerning bone, animal studies have shown that rosiglitazone induces bone loss by activating the protein peroxisome proliferator-activated receptor-gamma, a regulator of osteoblastic recruitment from the adipocytic lineage [112]. A cohort study showed that current use of glitazones in patients with T2D was associated with increased hip fracture risk with a more pronounced effect in women than in men [105]. Further, for rosiglitazone, clinical studies have shown increased bone resorption [113] and fracture risk in patients with T2D [114]. For pioglitazone, a randomized double-blind, placebo-controlled study found that treatment with pioglitazone significantly increased fracture risk compared with placebo [115]. The study was conducted in 3876 insulin-resistant patients without diabetes with a previous stroke or transient ischemic attack. A meta-analysis with 22 randomized controlled trials reported increased fracture risk with pioglitazone and rosiglitazone treatment in women but not in men [116], while another meta-analysis with six randomized trials found no significantly increased fracture risk with pioglitazone treatment. In summary, evidence suggests that both rosiglitazone and pioglitazone treatment are associated with bone loss and increased fracture risk.
5.5. Insulin
Insulin is the treatment of T1D and is used as additional treatment of T2D. Insulin is thought to act as an anabolic hormone on bone, evidence is based on the fact that patients with T1D have lower peak bone mass as a result of insulin deficiency. In general, insulin may impact fracture risk negatively by inducing hypoglycemia or positively by restoring normal levels of circulating glucose. Animal studies have shown that in mice lacking the insulin receptor substrates, bone formation is decreased, bone resorption increased [117], and bone turnover decreased [118]. For fracture risk, reports have been unclear with both evidence of a neutral effect on fracture risk [76], increased fracture risk [[119], [120], [121]], or decreased fracture risk [104].
5.6. Incretin-based therapy
For the newer incretin-based drugs, the glucagon-like peptide-1 receptor agonists (GLP-1 RA) and dipeptidylpeptidase 4 (DPP4)-inhibitors, interpretation of results from clinical studies are limited by a relative short follow-up period. For DPP4-inhibitors, an in vitro study suggested a direct negative effect on osteoclastogenesis by suppressing RANKL-mediated osteoclast differentiation and bone resorption [122]. A randomized clinical trial showed that one year treatment with the DPP4-inhibitor vildagliptin did not change bone turnover markers in drug-naïve patients with T2D [123]. A retrospective study showed that fracture risk in patients treated with DPP4-inhibitors was comparable to the fracture risk in treatment with either insulin or sulphonylurea drugs but lower than in treatment with glitazones [124], the latter probably due to increased fracture risk with glitazone treatment. Two retrospective studies showed decreased fracture risk with DPP4-inhibitor use compared to no use in patients with T2D [125,126], in both studies follow-up was maximally 5 years. Two other studies found neutral effect of DPP4-inhibitor treatment on fracture risk, a retrospective study [127], and a meta-analysis of randomized controlled trials [128]. For GLP-1 RAs, a number of animal studies have proposed a beneficial effect on bone turnover via inhibition of osteoclastogenesis [129]. A GLP-1-receptor knock-out mouse model showed that mice lacking the GLP-1 receptor developed cortical osteopenia, increased number of osteoclasts, and increased bone resorption activity compared with wild type mice [71]. A registry-based study showed no effect on fracture risk with GLP-1 RA treatment compared with other antidiabetic drugs [128]. Results from clinical studies on fracture risk with GLP-1 RA treatment are based on meta-analyses, and interpretation of the results are limited by a short follow up time and small numbers of fracture incidence. Most meta-analyses showed a neutral effect on fracture risk [130,131], but remarkably, one study found variable fracture risks depending on the type of GLP-1 RA drug [132]. A randomized controlled trial investigated the effect of Liraglutide in non-T2D obese women in a weight loss maintenance period, and reported that Liraglutide increased the levels of bone formation marker P1NP and prevented loss of bone mineral content compared with placebo [133].
5.7. Sodium-glucose cotransporter 2 inhibitors
The sodium-glucose co-transporter 2 (SGLT-2) inhibitors act via inhibition of glucose reabsorption in the kidney, thus increasing glucosuria, and decreasing blood glucose and weight. Concern has been raised, whether glucosuria may lead to disturbance of the calcium and phosphate homeostasis. So far, meta-analyses have reported no effect of SGLT-2 inhibitor use on fracture risk [134,135], but further longitudinal studies are warranted. Compared with some other antidiabetic drugs, the SGLT2 inhibitors would potentially reduce fracture risk as there is no risk of hypoglycemia and hence a lower risk of falls.
5.8. Bariatric surgery
Bone health in T2D patients treated with bariatric surgery has been shown to deteriorate. Surgery-treated patients experience increased bone turnover compared with nonoperated T2D patients, probably caused by inadequate absorption of vitamins and minerals, risk of secondary hyperparathyroidism, and a negative impact of weight-loss on bone, as review by Yu [136] for the different types of bariatric surgery. A recently published cross-sectional study compared the bone status of 96 previously Roux-En-Y gastric bypass (RYGB)-treated patients with 49 nonoperated patients with T2D [137]. The RYGB-treated patients had lower BMD, lower bone strength estimated by HRpQCT-derived vBMD and failure load, and increased levels of bone resorption and formation markers; CTX and P1NP, respectively, compared with the control group [137]. The results corroborate findings from another study [138]. Interestingly, the findings in the RYGB-treated patients were not associated with current diabetes status 6 years postsurgery [137]. However, as T2D is associated with low bone turnover and high BMD, the consequences of a lower BMD and higher bone turnover after bariatric surgery remains to be solved. Awareness should be raised that patients with T2D treated with bariatric surgery may suffer from detrimental skeletal changes, and we propose that these patients are followed up clinically concerning fracture risk and sufficient vitamin and mineral supplementation.
5.9. Other considerations
According to current guidelines from The American Diabetes Association, treatment of elderly patients with diabetes must allow for higher blood glucose levels to reduce the risk of hypoglycemia and the increased fall risk associated with hypoglycemia [139]. Treatment of T2D also include life-style interventions such as weight loss. Weight loss and reduced skeletal load may have harmful effects on bone and increase the risk of fragility fractures [140,141], and must be counteracted by weight-bearing exercise and adequate nutrition. See also section 2.3 for a further description of the relationship between weight/BMI and BMD.
6. Future perspectives
Despite the growing evidence that patients with T1D and T2D suffer from an increased fracture risk, there is still no clear consensus on how to clearly define or evaluate the impaired bone quality in diabetes. There is a demand for thorough longitudinal studies exploring the association between BMD and fracture risk in T1D and T2D and possible confounding by fracture site, age, sex, antidiabetic medication, blood glucose status, and diabetes duration. We speculate whether patients with T1D and T2D would benefit from having DXA routinely performed after the age of 50 years/menopause in women. Routine assessment of BMD would allow for intervention and fracture prevention, but currently specific data in patients with diabetes supporting this is lacking. Bone indentation and the assessment of bone material strength is an exciting new approach to bone quality and future studies will tell whether the method contributes to current knowledge on bone disease in diabetes.
7. Conclusion
Patients with T1D and T2D suffer from bone disease as a complication to diabetes. Bone turnover is decreased and bone microarchitecture is compromised leading to impaired bone quality. The underlying mechanisms for bone disease in T1D and T2D may be different due to different disease mechanisms. Common factors for patients with T1D and T2D are the increased risk of fracture, most predominantly in the hip, possible side-effects of antidiabetic medication, diagnostic challenges as conventional fracture predictors underestimate fracture risk, and questions regarding treatment of bone disease in diabetes as most currently used antiosteoporotic treatments lack evidence concerning the effect in patients with diabetes. While we are waiting for more evidence, we suggest assessing fracture risk in patients with diabetes by DXA and if available, biochemical markers of bone turnover.
Conflicts of interest
No potential conflict of interest relevant to this article was reported. ORCID. Katrine Hygum: 0000-0003-0411-995X. Jakob Starup-Linde: 0000-0002-4325-8023. Bente L. Langdahl: 0000-0002-8712-7199.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Bommer C., Heesemann E., Sagalova V., Manne-Goehler J., Atun R., Barnighausen T. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5:423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 2.Marshall D., Johnell O., Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis J.A., Oden A., Johansson H., Borgstrom F., Strom O., McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44:734–743. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- 4.Wang H., Ba Y., Xing Q., Du J.L. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y., Wei F., Lang Y., Liu Y. Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int. 2016;27:219–228. doi: 10.1007/s00198-015-3279-7. [DOI] [PubMed] [Google Scholar]
- 6.Shah V.N., Shah C.S., Snell-Bergeon J.K. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32:1134–1142. doi: 10.1111/dme.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janghorbani M., Van Dam R.M., Willett W.C., Hu F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 9.Jia P., Bao L., Chen H., Yuan J., Liu W., Feng F. Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int. 2017;28:3113–3121. doi: 10.1007/s00198-017-4183-0. [DOI] [PubMed] [Google Scholar]
- 10.Ni Y., Fan D. Diabetes mellitus is a risk factor for low bone mass-related fractures: a meta-analysis of cohort studies. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000008811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dytfeld J., Michalak M. Type 2 diabetes and risk of low-energy fractures in postmenopausal women: meta-analysis of observational studies. Aging Clin Exp Res. 2017;29:301–309. doi: 10.1007/s40520-016-0562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J., You W., Jing Z., Wang R., Fu Z., Wang Y. Increased risk of vertebral fracture in patients with diabetes: a meta-analysis of cohort studies. Int Orthop. 2016;40:1299–1307. doi: 10.1007/s00264-016-3146-y. [DOI] [PubMed] [Google Scholar]
- 13.Lipscombe L.L., Jamal S.A., Booth G.L., Hawker G.A. The risk of hip fractures in older individuals with diabetes: a population-based study. Diabetes Care. 2007;30:835–841. doi: 10.2337/dc06-1851. [DOI] [PubMed] [Google Scholar]
- 14.Leslie W.D., Lix L.M., Prior H.J., Derksen S., Metge C., O’Neil J. Biphasic fracture risk in diabetes: a population-based study. Bone. 2007;40:1595–1601. doi: 10.1016/j.bone.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz A.V., Sellmeyer D.E., Ensrud K.E., Cauley J.A., Tabor H.K., Schreiner P.J. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 17.Hough F.S., Pierroz D.D., Cooper C., Ferrari S.L. Mechanisms in endocrinology: mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174:R127–R138. doi: 10.1530/EJE-15-0820. [DOI] [PubMed] [Google Scholar]
- 18.Liao C.C., Lin C.S., Shih C.C., Yeh C.C., Chang Y.C., Lee Y.W. Increased risk of fracture and postfracture adverse events in patients with diabetes: two nationwide population-based retrospective cohort studies. Diabetes Care. 2014;37:2246–2252. doi: 10.2337/dc13-2957. [DOI] [PubMed] [Google Scholar]
- 19.Melton L.J., 3rd, Leibson C.L., Achenbach S.J., Therneau T.M., Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res. 2008;23:1334–1342. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majumdar S.R., Leslie W.D., Lix L.M., Morin S.N., Johansson H., Oden A. Longer duration of diabetes strongly impacts fracture risk assessment: the Manitoba BMD Cohort. J Clin Endocrinol Metab. 2016;101:4489–4496. doi: 10.1210/jc.2016-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee R.H., Sloane R., Pieper C., Lyles K.W., Adler R.A., Van Houtven C. Clinical fractures among older men with diabetes are mediated by diabetic complications. J Clin Endocrinol Metab. 2018;103:281–287. doi: 10.1210/jc.2017-01593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhukouskaya V.V., Eller-Vainicher C., Vadzianava V.V., Shepelkevich A.P., Zhurava I.V., Korolenko G.G. Prevalence of morphometric vertebral fractures in patients with type 1 diabetes. Diabetes Care. 2013;36:1635–1640. doi: 10.2337/dc12-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann T., Lodes S., Kästner B., Franke S., Kiehntopf M., Lehmann T. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int. 2014;25:1527–1533. doi: 10.1007/s00198-014-2631-7. [DOI] [PubMed] [Google Scholar]
- 24.Oei L., Zillikens M.C., Dehghan A., Buitendijk G.H., Castano-Betancourt M.C., Estrada K. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013;36:1619–1628. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vavanikunnel J., Charlier S., Becker C., Schneider C., Jick S.S., Meier C.R. Association between glycemic control and risk of fracture in diabetic patients: a nested case-control study. J Clin Endocrinol Metab. 2019;(104):1645–1654. doi: 10.1210/jc.2018-01879. [DOI] [PubMed] [Google Scholar]
- 26.Puar T.H., Khoo J.J., Cho L.W., Xu Y., Chen Y.T., Chuo A.M. Association between glycemic control and hip fracture. J Am Geriatr Soc. 2012;60:1493–1497. doi: 10.1111/j.1532-5415.2012.04052.x. [DOI] [PubMed] [Google Scholar]
- 27.Holmberg A.H., Nilsson P.M., Nilsson J.A., Akesson K. The association between hyperglycemia and fracture risk in middle age. A prospective, population-based study of 22,444 men and 10,902 women. J Clin Endocrinol Metab. 2008;93:815–822. doi: 10.1210/jc.2007-0843. [DOI] [PubMed] [Google Scholar]
- 28.Huang H.L., Pan C.C., Hsiao Y.F., Chen M.C., Kung C.Y., Kung P.T. Associations of body mass index and diabetes with hip fracture risk: a nationwide cohort study. BMC Public Health. 2018;18:1325. doi: 10.1186/s12889-018-6230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanazawa I., Notsu M., Takeno A., Tanaka K.I., Sugimoto T. Overweight and underweight are risk factors for vertebral fractures in patients with type 2 diabetes mellitus. J Bone Miner Metab. 2019;37:703–710. doi: 10.1007/s00774-018-0960-x. [DOI] [PubMed] [Google Scholar]
- 30.Davis W.A., Hamilton E.J., Bruce D.G., Davis T.M.E. Development and validation of a simple hip fracture risk prediction tool for type 2 diabetes: the Fremantle Diabetes Study Phase I. Diabetes Care. 2019;42:102–109. doi: 10.2337/dc18-1486. [DOI] [PubMed] [Google Scholar]
- 31.Kanazawa I., Tanaka K.I., Takeo A., Notsu M., Miyake H., Sugimoto T. A scoring assessment tool for the risk of vertebral fractures in patients with type 2 diabetes mellitus. Bone. 2019;122:38–44. doi: 10.1016/j.bone.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Li G., Prior J.C., Leslie W.D., Thabane L., Papaioannou A., Josse R.G. Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care. 2019;42:507–513. doi: 10.2337/dc18-1965. [DOI] [PubMed] [Google Scholar]
- 33.Furtado S., Rodrigues A., Dias S., Branco J.C., Canhao H. Self-reported low-energy fractures and associated risk factors in people with diabetes: a national population-based study. Diabetes Res Clin Pract. 2019;147:93–101. doi: 10.1016/j.diabres.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjoblom S., Suuronen J., Rikkonen T., Honkanen R., Kroger H., Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75:175–180. doi: 10.1016/j.maturitas.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Kim T.N., Park M.S., Yang S.J., Yoo H.J., Kang H.J., Song W. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerrero N., Bunout D., Hirsch S., Barrera G., Leiva L., Henriquez S. Premature loss of muscle mass and function in type 2 diabetes. Diabetes Res Clin Pract. 2016;117:32–38. doi: 10.1016/j.diabres.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Hasserius R., Karlsson M.K., Jonsson B., Redlund-Johnell I., Johnell O. Long-term morbidity and mortality after a clinically diagnosed vertebral fracture in the elderly--a 12- and 22-year follow-up of 257 patients. Calcif Tissue Int. 2005;76:235–242. doi: 10.1007/s00223-004-2222-2. [DOI] [PubMed] [Google Scholar]
- 39.Panula J., Pihlajamaki H., Mattila V.M., Jaatinen P., Vahlberg T., Aarnio P. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Muscoskelet Disord. 2011;12:105. doi: 10.1186/1471-2474-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulcelik N.E., Bayraktar M., Caglar O., Alpaslan M., Karakaya J. Mortality after hip fracture in diabetic patients. Exp Clin Endocrinol Diabetes. 2011;119:414–418. doi: 10.1055/s-0030-1270466. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Huedo M.A., Jimenez-Garcia R., Mora-Zamorano E., Hernandez-Barrera V., Villanueva-Martinez M., Lopez-de-Andres A. Trends in incidence of proximal humerus fractures, surgical procedures and outcomes among elderly hospitalized patients with and without type 2 diabetes in Spain (2001-2013) BMC Muscoskelet Disord. 2017;18:522. doi: 10.1186/s12891-017-1892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nirantharakumar K., Toulis K.A., Wijesinghe H., Mastan M.S., Srikantharajah M., Bhatta S. Impact of diabetes on inpatient mortality and length of stay for elderly patients presenting with fracture of the proximal femur. J Diabet Complicat. 2013;27:208–210. doi: 10.1016/j.jdiacomp.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Voko Z., Gaspar K., Inotai A., Horvath C., Bors K., Speer G. Osteoporotic fractures may impair life as much as the complications of diabetes. J Eval Clin Pract. 2017;23:1375–1380. doi: 10.1111/jep.12800. [DOI] [PubMed] [Google Scholar]
- 44.Krakauer J.C., McKenna M.J., Buderer N.F., Rao D.S., Whitehouse F.W., Parfitt A.M. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 45.Manavalan J.S., Cremers S., Dempster D.W., Zhou H., Dworakowski E., Kode A. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:3240–3250. doi: 10.1210/jc.2012-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armas L.A., Akhter M.P., Drincic A., Recker R.R. Trabecular bone histomorphometry in humans with type 1 diabetes mellitus. Bone. 2012;50:91–96. doi: 10.1016/j.bone.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hygum K., Starup-Linde J., Harslof T., Vestergaard P., Langdahl B.L. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:R137–R157. doi: 10.1530/EJE-16-0652. [DOI] [PubMed] [Google Scholar]
- 48.Wijenayaka A.R., Kogawa M., Lim H.P., Bonewald L.F., Findlay D.M., Atkins G.J. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitase Y., Barragan L., Qing H., Kondoh S., Jiang J.X., Johnson M.L. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raska I., Jr., Raskova M., Zikan V., Skrha J. Prevalence and risk factors of osteoporosis in postmenopausal women with type 2 diabetes mellitus. Cent Eur J Publ Health. 2017;25:3–10. doi: 10.21101/cejph.a4717. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka K., Yamaguchi T., Kanazawa I., Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461:193–199. doi: 10.1016/j.bbrc.2015.02.091. [DOI] [PubMed] [Google Scholar]
- 52.Kang J., Boonanantanasarn K., Baek K., Woo K.M., Ryoo H.M., Baek J.H. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. Journal of periodontal & implant science. 2015;45:101–110. doi: 10.5051/jpis.2015.45.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniele G., Winnier D., Mari A., Bruder J., Fourcaudot M., Pengou Z. Sclerostin and insulin resistance in prediabetes: evidence of a cross talk between bone and glucose metabolism. Diabetes Care. 2015;38:1509–1517. doi: 10.2337/dc14-2989. [DOI] [PubMed] [Google Scholar]
- 54.Yang D.H., Chiang T.I., Chang I.C., Lin F.H., Wei C.C., Cheng Y.W. Increased levels of circulating advanced glycation end-products in menopausal women with osteoporosis. Int J Med Sci. 2014;11:453–460. doi: 10.7150/ijms.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerkeni M., Saidi A., Bouzidi H., Ben Yahya S., Hammami M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc Res. 2012;84:378–383. doi: 10.1016/j.mvr.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Valcourt U., Merle B., Gineyts E., Viguet-Carrin S., Delmas P.D., Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007;282:5691–5703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- 57.Sanguineti R., Storace D., Monacelli F., Federici A., Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci. 2008;1126:166–172. doi: 10.1196/annals.1433.044. [DOI] [PubMed] [Google Scholar]
- 58.Katayama Y., Akatsu T., Yamamoto M., Kugai N., Nagata N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res. 1996;11:931–937. doi: 10.1002/jbmr.5650110709. [DOI] [PubMed] [Google Scholar]
- 59.Faerch K., Torekov S.S., Vistisen D., Johansen N.B., Witte D.R., Jonsson A. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO Study. Diabetes. 2015;64:2513–2525. doi: 10.2337/db14-1751. [DOI] [PubMed] [Google Scholar]
- 60.Toft-Nielsen M.B., Damholt M.B., Madsbad S., Hilsted L.M., Hughes T.E., Michelsen B.K. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 61.Muscelli E., Mari A., Casolaro A., Camastra S., Seghieri G., Gastaldelli A. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–1348. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 62.Greenbaum C.J., Prigeon R.L., D’Alessio D.A. Impaired beta-cell function, incretin effect, and glucagon suppression in patients with type 1 diabetes who have normal fasting glucose. Diabetes. 2002;51:951–957. doi: 10.2337/diabetes.51.4.951. [DOI] [PubMed] [Google Scholar]
- 63.Vilsboll T., Krarup T., Sonne J., Madsbad S., Volund A., Juul A.G. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 64.Lugari R., Dell’Anna C., Ugolotti D., Dei Cas A., Barilli A.L., Zandomeneghi R. Effect of nutrient ingestion on glucagon-like peptide 1 (7-36 amide) secretion in human type 1 and type 2 diabetes. Horm Metab Res. 2000;32:424–428. doi: 10.1055/s-2007-978665. [DOI] [PubMed] [Google Scholar]
- 65.Clowes J.A., Allen H.C., Prentis D.M., Eastell R., Blumsohn A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab. 2003;88:4867–4873. doi: 10.1210/jc.2002-021447. [DOI] [PubMed] [Google Scholar]
- 66.Bollag R.J., Zhong Q., Phillips P., Min L., Zhong L., Cameron R. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141:1228–1235. doi: 10.1210/endo.141.3.7366. [DOI] [PubMed] [Google Scholar]
- 67.Pacheco-Pantoja E.L., Ranganath L.R., Gallagher J.A., Wilson P.J., Fraser W.D. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12 doi: 10.1186/1472-6793-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong Q., Itokawa T., Sridhar S., Ding K.H., Xie D., Kang B. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab. 2007;292:E543–E548. doi: 10.1152/ajpendo.00364.2006. [DOI] [PubMed] [Google Scholar]
- 69.Nuche-Berenguer B., Portal-Nunez S., Moreno P., Gonzalez N., Acitores A., Lopez-Herradon A. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010;225:585–592. doi: 10.1002/jcp.22243. [DOI] [PubMed] [Google Scholar]
- 70.Mabilleau G., Mieczkowska A., Irwin N., Flatt P.R., Chappard D. Optimal bone mechanical and material properties require a functional glucagon-like peptide-1 receptor. J Endocrinol. 2013;219:59–68. doi: 10.1530/JOE-13-0146. [DOI] [PubMed] [Google Scholar]
- 71.Yamada C., Yamada Y., Tsukiyama K., Yamada K., Udagawa N., Takahashi N. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149:574–579. doi: 10.1210/en.2007-1292. [DOI] [PubMed] [Google Scholar]
- 72.Shanbhogue V.V., Hansen S., Frost M., Brixen K., Hermann A.P. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 2017;5:827–838. doi: 10.1016/S2213-8587(17)30134-1. [DOI] [PubMed] [Google Scholar]
- 73.Kim J.H., Jung M.H., Lee J.M., Son H.S., Cha B.Y., Chang S.A. Diabetic peripheral neuropathy is highly associated with nontraumatic fractures in Korean patients with type 2 diabetes mellitus. Clin Endocrinol. 2012;77:51–55. doi: 10.1111/j.1365-2265.2011.04222.x. [DOI] [PubMed] [Google Scholar]
- 74.Ivers R.Q., Cumming R.G., Mitchell P., Peduto A.J. Diabetes and risk of fracture: the blue mountains eye study. Diabetes Care. 2001;24:1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 75.Viegas M., Costa C., Lopes A., Griz L., Medeiro M.A., Bandeira F. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabet Complicat. 2011;25:216–221. doi: 10.1016/j.jdiacomp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 76.Starup-Linde J., Gregersen S., Vestergaard P. Associations with fracture in patients with diabetes: a nested case-control study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vestergaard P., Rejnmark L., Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif Tissue Int. 2009;84:45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 78.Bonds D.E., Larson J.C., Schwartz A.V., Strotmeyer E.S., Robbins J., Rodriguez B.L. Risk of fracture in women with type 2 diabetes: the women’s health initiative observational study. J Clin Endocrinol Metab. 2006;91:3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz A.V., Vittinghoff E., Bauer D.C., Hillier T.A., Strotmeyer E.S., Ensrud K.E. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. J Am Med Assoc. 2011;305:2184–2192. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fraser L.A., Pritchard J., Ioannidis G., Giangegorio L.M., Adachi J.D., Papaioannou A. Clinical risk factors for fracture in diabetes: a matched cohort analysis. J Clin Densitom. 2011;14:416–421. doi: 10.1016/j.jocd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giangregorio L.M., Leslie W.D., Lix L.M., Johansson H., Oden A., McCloskey E. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27:301–308. doi: 10.1002/jbmr.556. [DOI] [PubMed] [Google Scholar]
- 82.Samelson E.J., Broe K.E., Xu H., Yang L., Boyd S., Biver E. Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol. 2019;7:34–43. doi: 10.1016/S2213-8587(18)30308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bridges D., Randall C., Hansma P.K. A new device for performing reference point indentation without a reference probe. Rev Sci Instrum. 2012;83 doi: 10.1063/1.3693085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Randall C., Bridges D., Guerri R., Nogues X., Puig L., Torres E. Applications of a new handheld reference point indentation instrument measuring bone material strength. J Med Dev. 2013;7:410051–410056. doi: 10.1115/1.4024829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samelson E.J., Demissie S., Cupples L.A., Zhang X., Xu H., Liu C.T. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: framingham HR-pQCT study. J Bone Miner Res. 2018;33:54–62. doi: 10.1002/jbmr.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nilsson A.G., Sundh D., Johansson L., Nilsson M., Mellstrom D., Rudang R. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res. 2017;32:1062–1071. doi: 10.1002/jbmr.3057. [DOI] [PubMed] [Google Scholar]
- 87.Starup-Linde J., Lykkeboe S., Gregersen S., Hauge E.M., Langdahl B.L., Handberg A. Bone structure and predictors of fracture in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2016;101:928–936. doi: 10.1210/jc.2015-3882. [DOI] [PubMed] [Google Scholar]
- 88.Shanbhogue V.V., Hansen S., Frost M., Jorgensen N.R., Hermann A.P., Henriksen J.E. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in adult patients with type 1 diabetes mellitus. J Bone Miner Res. 2015;30:2188–2199. doi: 10.1002/jbmr.2573. [DOI] [PubMed] [Google Scholar]
- 89.Farr J.N., Drake M.T., Amin S., Melton L.J., 3rd, McCready L.K., Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29:787–795. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leslie W.D., Aubry-Rozier B., Lamy O., Hans D. TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 91.Dhaliwal R., Cibula D., Ghosh C., Weinstock R.S., Moses A.M. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014;25:1969–1973. doi: 10.1007/s00198-014-2704-7. [DOI] [PubMed] [Google Scholar]
- 92.Kim J.H., Choi H.J., Ku E.J., Kim K.M., Kim S.W., Cho N.H. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100:475–482. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- 93.Ferrari S.L., Abrahamsen B., Napoli N., Akesson K., Chandran M., Eastell R. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int. 2018;29:2285–2596. doi: 10.1007/s00198-018-4650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Staa T.P., Laan R.F., Barton I.P., Cohen S., Reid D.M., Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 95.Keegan T.H., Schwartz A.V., Bauer D.C., Sellmeyer D.E., Kelsey J.L. Fracture intervention t. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004;27:1547–1553. doi: 10.2337/diacare.27.7.1547. [DOI] [PubMed] [Google Scholar]
- 96.Abrahamsen B., Rubin K.H., Eiken P.A., Eastell R. Characteristics of patients who suffer major osteoporotic fractures despite adhering to alendronate treatment: a National Prescription registry study. Osteoporos Int. 2013;24:321–328. doi: 10.1007/s00198-012-2184-6. [DOI] [PubMed] [Google Scholar]
- 97.Vestergaard P., Rejnmark L., Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int. 2011;88:209–214. doi: 10.1007/s00223-010-9450-4. [DOI] [PubMed] [Google Scholar]
- 98.Inoue D., Muraoka R., Okazaki R., Nishizawa Y., Sugimoto T. Efficacy and safety of risedronate in osteoporosis subjects with comorbid diabetes, hypertension, and/or dyslipidemia: a post hoc analysis of phase III trials conducted in Japan. Calcif Tissue Int. 2016;98:114–122. doi: 10.1007/s00223-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schwartz A.V., Pavo I., Alam J., Disch D.P., Schuster D., Harris J.M. Teriparatide in patients with osteoporosis and type 2 diabetes. Bone. 2016;91:152–158. doi: 10.1016/j.bone.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y., Cao Y., Zhang S., Zhang W., Zhang B., Tang Q. Romosozumab treatment in postmenopausal women with osteoporosis: a meta-analysis of randomized controlled trials. Climacteric. 2018;21:189–195. doi: 10.1080/13697137.2018.1433655. [DOI] [PubMed] [Google Scholar]
- 101.Molinuevo M.S., Schurman L., McCarthy A.D., Cortizo A.M., Tolosa M.J., Gangoiti M.V. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res. 2010;25:211–221. doi: 10.1359/jbmr.090732. [DOI] [PubMed] [Google Scholar]
- 102.Cortizo A.M., Sedlinsky C., McCarthy A.D., Blanco A., Schurman L. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol. 2006;536:38–46. doi: 10.1016/j.ejphar.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 103.Stage T.B., Christensen M.H., Jorgensen N.R., Beck-Nielsen H., Brosen K., Gram J. Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone. 2018;112:35–41. doi: 10.1016/j.bone.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 104.Vestergaard P., Rejnmark L., Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 105.Starup-Linde J., Gregersen S., Frost M., Vestergaard P. Use of glucose-lowering drugs and risk of fracture in patients with type 2 diabetes. Bone. 2017;95:136–142. doi: 10.1016/j.bone.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 106.Colhoun H.M., Livingstone S.J., Looker H.C., Morris A.D., Wild S.H., Lindsay R.S. Hospitalised hip fracture risk with rosiglitazone and pioglitazone use compared with other glucose-lowering drugs. Diabetologia. 2012;55:2929–2937. doi: 10.1007/s00125-012-2668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Napoli N., Strotmeyer E.S., Ensrud K.E., Sellmeyer D.E., Bauer D.C., Hoffman A.R. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014;57:2057–2065. doi: 10.1007/s00125-014-3289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fronczek-Sokol J., Pytlik M. Effect of glimepiride on the skeletal system of ovariectomized and non-ovariectomized rats. Pharmacol Rep. 2014;66:412–417. doi: 10.1016/j.pharep.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 109.Rajpathak S.N., Fu C., Brodovicz K.G., Engel S.S., Lapane K. Sulfonylurea use and risk of hip fractures among elderly men and women with type 2 diabetes. Drugs Aging. 2015;32:321–327. doi: 10.1007/s40266-015-0254-0. [DOI] [PubMed] [Google Scholar]
- 110.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 111.Tang H., Shi W., Fu S., Wang T., Zhai S., Song Y. Pioglitazone and bladder cancer risk: a systematic review and meta-analysis. Cancer Med. 2018;7:1070–1080. doi: 10.1002/cam4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lazarenko O.P., Rzonca S.O., Hogue W.R., Swain F.L., Suva L.J., Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harslof T., Wamberg L., Moller L., Stodkilde-Jorgensen H., Ringgaard S., Pedersen S.B. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab. 2011;96:1541–1548. doi: 10.1210/jc.2010-2077. [DOI] [PubMed] [Google Scholar]
- 114.Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 115.Viscoli C.M., Inzucchi S.E., Young L.H., Insogna K.L., Conwit R., Furie K.L. Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab. 2017;102:914–922. doi: 10.1210/jc.2016-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu Z.N., Jiang Y.F., Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115–123. doi: 10.1016/j.bone.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 117.Akune T., Ogata N., Hoshi K., Kubota N., Terauchi Y., Tobe K. Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts. J Cell Biol. 2002;159:147–156. doi: 10.1083/jcb.200204046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ogata N., Chikazu D., Kubota N., Terauchi Y., Tobe K., Azuma Y. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Investig. 2000;105:935–943. doi: 10.1172/JCI9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wallander M., Axelsson K.F., Nilsson A.G., Lundh D., Lorentzon M. Type 2 diabetes and risk of hip fractures and non-skeletal fall injuries in the elderly: a study from the Fractures and Fall Injuries in the Elderly Cohort (FRAILCO) J Bone Miner Res. 2017;32:449–460. doi: 10.1002/jbmr.3002. [DOI] [PubMed] [Google Scholar]
- 120.Losada E., Soldevila B., Ali M.S., Martinez-Laguna D., Nogues X., Puig-Domingo M. Real-world antidiabetic drug use and fracture risk in 12,277 patients with type 2 diabetes mellitus: a nested case-control study. Osteoporos Int. 2018;29:2079–2086. doi: 10.1007/s00198-018-4581-y. [DOI] [PubMed] [Google Scholar]
- 121.Losada-Grande E., Hawley S., Soldevila B., Martinez-Laguna D., Nogues X., Diez-Perez A. Insulin use and excess fracture risk in patients with type 2 diabetes: a propensity-matched cohort analysis. Sci Rep. 2017;7:3781. doi: 10.1038/s41598-017-03748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang C., Xiao F., Qu X., Zhai Z., Hu G., Chen X. Sitagliptin, an anti-diabetic drug, suppresses estrogen deficiency-induced osteoporosisin vivo and inhibits RANKL-induced osteoclast formation and bone resorption in vitro. Front Pharmacol. 2017;8:407. doi: 10.3389/fphar.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bunck M.C., Poelma M., Eekhoff E.M., Schweizer A., Heine R.J., Nijpels G. Effects of vildagliptin on postprandial markers of bone resorption and calcium homeostasis in recently diagnosed, well-controlled type 2 diabetes patients. J Diabetes. 2012;4:181–185. doi: 10.1111/j.1753-0407.2011.00168.x. [DOI] [PubMed] [Google Scholar]
- 124.Gamble J.M., Donnan J.R., Chibrikov E., Twells L.K., Midodzi W.K., Majumdar S.R. The risk of fragility fractures in new users of dipeptidyl peptidase-4 inhibitors compared to sulfonylureas and other anti-diabetic drugs: a cohort study. Diabetes Res Clin Pract. 2018;136:159–167. doi: 10.1016/j.diabres.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 125.Dombrowski S., Kostev K., Jacob L. Use of dipeptidyl peptidase-4 inhibitors and risk of bone fracture in patients with type 2 diabetes in Germany-A retrospective analysis of real-world data. Osteoporos Int. 2017;28:2421–2428. doi: 10.1007/s00198-017-4051-y. [DOI] [PubMed] [Google Scholar]
- 126.Hou W.H., Chang K.C., Li C.Y., Ou H.T. Dipeptidyl peptidase-4 inhibitor use is associated with decreased risk of fracture in patients with type 2 diabetes: a population-based cohort study. Br J Clin Pharmacol. 2018;84:2029–2039. doi: 10.1111/bcp.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Driessen J.H., van den Bergh J.P., van Onzenoort H.A., Henry R.M., Leufkens H.G., de Vries F. Long-term use of dipeptidyl peptidase-4 inhibitors and risk of fracture: a retrospective population-based cohort study. Diabetes Obes Metab. 2017;19:421–428. doi: 10.1111/dom.12843. [DOI] [PubMed] [Google Scholar]
- 128.Driessen J.H., van Onzenoort H.A., Starup-Linde J., Henry R., Neef C., van den Bergh J. Use of dipeptidyl peptidase 4 inhibitors and fracture risk compared to use of other anti-hyperglycemic drugs. Pharmacoepidemiol Drug Saf. 2015;24:1017–1025. doi: 10.1002/pds.3837. [DOI] [PubMed] [Google Scholar]
- 129.Wen B., Zhao L., Zhao H., Wang X. Liraglutide exerts a bone-protective effect in ovariectomized rats with streptozotocin-induced diabetes by inhibiting osteoclastogenesis. Exp Ther Med. 2018;15:5077–5083. doi: 10.3892/etm.2018.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Driessen J.H., de Vries F., van Onzenoort H., Harvey N.C., Neef C., van den Bergh J.P. The use of incretins and fractures - a meta-analysis on population-based real life data. Br J Clin Pharmacol. 2017;83:923–926. doi: 10.1111/bcp.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mabilleau G., Mieczkowska A., Chappard D. Use of glucagon-like peptide-1 receptor agonists and bone fractures: a meta-analysis of randomized clinical trials. J Diabetes. 2014;6:260–266. doi: 10.1111/1753-0407.12102. [DOI] [PubMed] [Google Scholar]
- 132.Su B., Sheng H., Zhang M., Bu L., Yang P., Li L. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: a meta-analysis of randomized controlled trials. Endocrine. 2015;48:107–115. doi: 10.1007/s12020-014-0361-4. [DOI] [PubMed] [Google Scholar]
- 133.Iepsen E.W., Lundgren J.R., Hartmann B., Pedersen O., Hansen T., Jorgensen N.R. GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab. 2015;100:2909–2917. doi: 10.1210/jc.2015-1176. [DOI] [PubMed] [Google Scholar]
- 134.Tang H.L., Li D.D., Zhang J.J., Hsu Y.H., Wang T.S., Zhai S.D. Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2016;18:1199–1206. doi: 10.1111/dom.12742. [DOI] [PubMed] [Google Scholar]
- 135.Kohler S., Kaspers S., Salsali A., Zeller C., Woerle H.J. Analysis of fractures in patients with type 2 diabetes treated with empagliflozin in pooled data from placebo-controlled trials and a head-to-head study versus glimepiride. Diabetes Care. 2018;41:1809–1816. doi: 10.2337/dc17-1525. [DOI] [PubMed] [Google Scholar]
- 136.Yu E.W. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29:1507–1518. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Madsen L.R., Espersen R., Ornstrup M.J., Jorgensen N.R., Langdahl B.L., Richelsen B. Bone health in patients with type 2 diabetes treated by roux-en-y gastric bypass and the role of diabetes remission. Obes Surg. 2019 Feb 4 doi: 10.1007/s11695-019-03753-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 138.Lindeman K.G., Greenblatt L.B., Rourke C., Bouxsein M.L., Finkelstein J.S., Yu E.W. Longitudinal 5-year evaluation of bone density and microarchitecture after roux-en-y gastric bypass surgery. J Clin Endocrinol Metab. 2018;103:4104–4112. doi: 10.1210/jc.2018-01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.American Diabetes Association Summary of revisions: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S4–S6. [Google Scholar]
- 140.Johnson K.C., Bray G.A., Cheskin L.J., Clark J.M., Egan C.M., Foreyt J.P. The effect of intentional weight loss on fracture risk in persons with diabetes: results from the look AHEAD randomized clinical trial. J Bone Miner Res. 2017;32:2278–2287. doi: 10.1002/jbmr.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Komorita Y., Iwase M., Fujii H., Ohkuma T., Ide H., Jodai-Kitamura T. Impact of body weight loss from maximum weight on fragility bone fractures in Japanese patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care. 2018;41:1061–1067. doi: 10.2337/dc17-2004. [DOI] [PubMed] [Google Scholar]