Abstract
Objectives
We examined whether eldecalcitol (ELD) provided additive bone mineral density (BMD) and bone turnover marker gains in patients undergoing long-term bisphosphonate (BP) usage, especially in osteoporotic individuals exhibiting a poor response to BPs.
Methods
Forty-two post-menopausal patients with primary osteoporosis and low lumbar BMD (L-BMD) and/or bilateral total hip BMD (H-BMD) values receiving long-term BP treatment were prospectively enrolled. Serum bone alkaline phosphatase (BAP) was measured as a bone formation marker and urinary N-terminal telopeptide of type I collagen (NTX) was assessed as a bone resorption marker. L-BMD, H-BMD, and femoral neck BMD (N-BMD) were recorded before, at the commencement of, and during ELD administration.
Results
BAP and urinary NTX were significantly decreased by BP therapy prior to ELD. ELD addition further significantly decreased the bone turnover markers (both p < 0.01). The mean L-BMD increase rate was 0.2% (p = 0.81) from 2 to 1 years before ELD administration, −0.7% (p = 0.30) during the year before ELD, and 2.9% (p < 0.01) during 1 year of ELD. Similar findings were observed for the mean increase rate of H-BMD, with values of 0.2% (p = 0.55), −0.7% (p < 0.01), and 1.2% (p < 0.01), respectively. The mean N-BMD increase rate was significantly increased after ELD administration (1.1%, p = 0.03) despite no gains by BP therapy alone.
Conclusions
This study suggests that ELD addition may be useful for osteoporotic patients exhibiting a diminished long-term BP therapy response.
Keywords: Bisphosphonate, Bone mineral density, Eldecalcitol, Osteoporosis
1. Introduction
Osteoporosis (OP) is a widespread skeletal disorder requiring long-term management to prevent fractures, maintain activities of daily living, and ultimately reduce mortality. Bisphosphonates (BPs) are the most common drugs for OP. With their bone anti-resorptive properties, BPs improve bone turnover, increase bone mineral density (BMD), and decrease fracture incidence [1,2]. Nitrogen-containing BPs inhibit farnesyl pyrophosphate synthetase in the mevalonate pathway in osteoclasts, [3] thereby suppressing their function and modulating osteoclast activity.
Vitamin D is essential for maintaining bone and calcium (Ca) metabolism. While circulating serum 25-hydroxyvitamin D3 (25[OH]D3) is the major and main storage form of vitamin D in the human body, active vitamin D (1,25-dihydroxycholecalciferol [1,25(OH)2D3]) regulates bone and Ca metabolism. As a vitamin D analog, 1-α hydroxycholecalciferol (1α[OH]D3; alfacalcidol [ALF]) has been approved for OP treatment in Japan [4] and is frequently employed in disease management to modulate serum 1,25(OH)2D3 and parathyroid hormone levels without affecting bone turnover markers [5]. Combination therapy of bone antiresorptive drugs and ALF exhibited additive antiosteoporotic effects when compared with anti-resorptive drug monotherapy in postmenopausal women [5,6]. Recently, 1α,25(OH)2-2β-(3-hydroxypropyloxy)D3 (eldecalcitol; ELD) has been introduced in OP treatment as a newly developed analog of active vitamin D [7,8] that inhibits bone resorption through the disruption of osteoclast formation. In a trial of Japanese patients with OP, ELD increased BMD and decreased the incidence of vertebral fractures more effectively than did ALF [8]. Moreover, a combination of bone antiresorptive drugs and ELD produced enhanced antiosteoporotic effects over non-ELD monotherapy in postmenopausal women [9].
The BMD gains imparted by BPs in OP are especially prominent during the first few years of treatment. However, drug effectiveness can diminish over longer treatment periods [10], and BMD plateaus and even decreases have been encountered regardless of the BP usage. We previously reported that in BP poor-response patients, many of whom receiving therapy for over 5 years, BMD decreased significantly over time [11]. In such cases, alternative treatment is required.
We earlier described that BP therapy combined with ELD was more effective for OP treatment than when combined with ALF since ELD subsequent to ALF significantly suppressed bone turnover and increased lumbar BMD (L-BMD), [12] suggesting a superiority of combination BP therapy with ELD even in BP poor-response patients. To our knowledge, no reports have addressed the additive effects of ELD on poor responders to long-term BPs until the current investigation.
2. Patients and methods
2.1. Patient recruitment and diagnosis of OP
Patient demographic data are summarized in Table 1. Only postmenopausal female patients were enrolled in this prospective study. During the period from November 2014 to August 2017, we recruited 46 BP response-poor patients with primary OP and low L-BMD and/or total hip BMD (H-BMD) values undergoing long-term BP therapy. A total of 42 subjects were enrolled after 4 patients were excluded due to insufficient data collection during observation. We defined poor BP responders as individuals in whom L-BMD or H-BMD did not apparently increase with chronic BP administration over a 2-year period. In 21 of 42 patients, bone turnover markers were measured before and after ELD addition. The diagnosis of primary OP in this study was made in accordance with the revised criteria established by the Japanese Society of Bone and Mineral Research [13].
Table 1.
Patient characteristics (n=42)
| Characteristic | Mean ± SD |
|---|---|
| Age, yr | 72.9 ± 7.3 |
| Height, cm | 152.9 ± 5.4 |
| Weight, kg | 50.8 ± 7.3 |
| Pre-treatment period of BP, mo | 61.9 ± 30.3 |
| Albumin corrected calcium, mg/dL | 9.1 ± 0.3 |
| Phosphorus, mg/dL | 3.3 ± 0.4 |
| Creatinine, mg/dL | 0.62 ± 0.10 |
SD, standard deviation; BP: bisphosphonate.
2.2. Inclusion and exclusion criteria for this study
The inclusion criteria for this study were postmenopausal Japanese women with primary OP. The exclusion criteria were the presence of obvious complications, such as chronic renal failure, bone metabolic disorders, liver dysfunction, and diabetes mellitus, all of which might affect OP. Serum renal and hepatic enzymes were within normal ranges before BP administration in all patients.
2.3. Drug selection
Alendronate, risedronate, and minodronate were adopted in various regimens during the long-term BP pretreatment. We did not examine the effects of individual BPs since they were often switched for patients exhibiting poor responsiveness. All participants took daily oral ELD of 0.75 μg/day after breakfast during the study period. All patients received BPs without Ca or vitamin D supplementation before and during ELD administration.
2.4. Measurement of bone turnover markers
Serum bone alkaline phosphatase (BAP) (Beckman Coulter, Inc., Tokyo, Japan) was measured as a bone formation marker using a chemiluminescent enzyme immunoassay with inter- and intra-assay coefficients of variation (CVs) of 3.0% and 2.5%, respectively. Urinary N-terminal telopeptide (NTX) of type I collagen (Osteomark; Osteox International, Seattle, WA, USA) was assessed as a marker of bone resorption using an enzyme-linked immunosorbent assay with inter- and intra-assay CVs of 11.5% and 12.7%, respectively. Each marker was measured before BP administration, just prior to ELD addition, and at 4 months of ELD administration. After overnight fasting, serum and first-void urine samples were collected between 8:30 a.m. and 10:00 a.m. Immunoassays were performed by SRL, Inc. (Tokyo, Japan).
2.5. Measurement of BMD
BMD was measured using a dual-energy X-ray absorption fan-beam bone densitometer (Lunar Prodigy; GE Healthcare Bio-Sciences Corp., Little Chalfont, UK) at the lumbar 1–4 level of the posteroanterior spine and at the bilateral hips as the mean of the right and left sides. Fracture sites were avoided for BMD evaluation. The CVs of BMD measurement at the lumbar spine, total hip, and femoral neck were 0.8%, 0.7%, and 1.2%, respectively. The respective least significant changes of these measurements were 2.3%, 1.8%, and 3.3% [14,15].
2.6. Statistical analysis
For statistical analysis, comparisons of markers and BMD at each measurement point were conducted using paired t-tests with Bonferroni correction. Results were expressed as the mean ± standard deviation. Annual BMD change rates were analyzed using one-sample t-tests. Statistical analyses were performed using the statistical package R, version 3.5.1 (available at http://www.r-project.org). A P-value of <0.05 was considered statistically significant.
2.7. Ethical approval
This investigation was performed in accordance with the ethical tenets set forth in the revised 2014 Declaration of Helsinki. The study was approved by the Institutional Ethics Committee of Shinshu University School of Medicine (Protocol No. 2014-22). Written informed consent was obtained from all patients.
3. Results
The cohort's demographic characteristics are presented in Table 1. Fig. 1, Fig. 2 summarizes the changes in bone turnover markers. Fig. 3, Fig. 4 respectively show the values and percent changes in BMD.
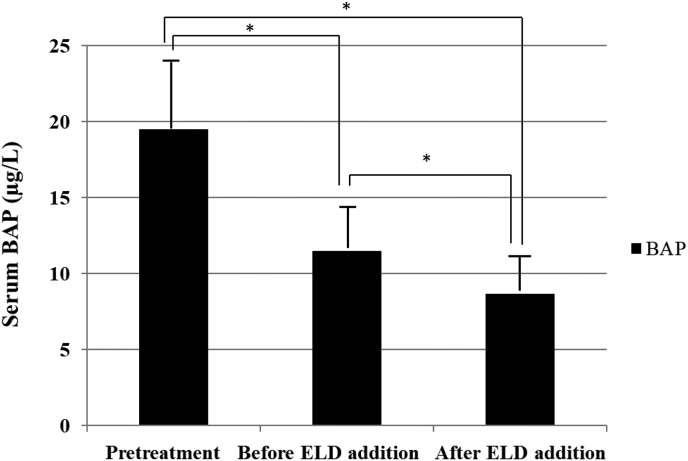
Fig. 1.
Changes in serum bone alkaline phosphatase (BAP) before bisphosphonate (BP) treatment, before eldecalcitol (ELD) addition, and at 4 months after ELD addition. Prior to BP treatment, mean serum BAP was 19.5 ± 9.6U/L, which had decreased significantly to 11.5 ± 3.6U/L before ELD addition (P< 0.01). With ELD, BAP further decreased significantly to 8.7 ± 2.2U/L (P< 0.01). *P < 0.05.
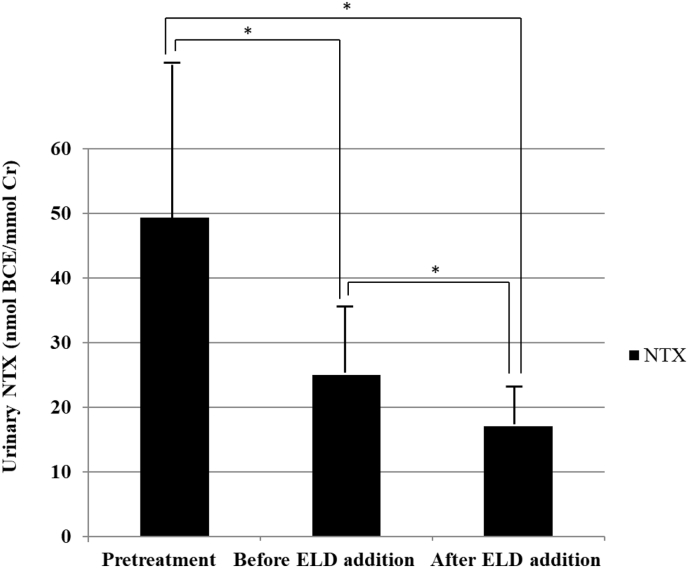
Fig. 2.
Changes in urinary N-terminal telopeptide of type I collagen (NTX) before bisphosphonate (BP) treatment, before eldecalcitol (ELD) addition, and at 4 months after ELD addition. Before BP therapy, mean urinary NTX was 49.3 ± 25.9 nmol bone collagen equivalent (BCE)/mmol creatinine (Cr). This had decreased significantly to 25.0 ± 10.6 nmol BCE/mmol Cr prior to ELD commencement (P< 0.01) and was further significantly reduced after the addition of ELD to 17.0 ± 5.9 nmol BCE/mmol Cr (P= 0.01). *P < 0.05.
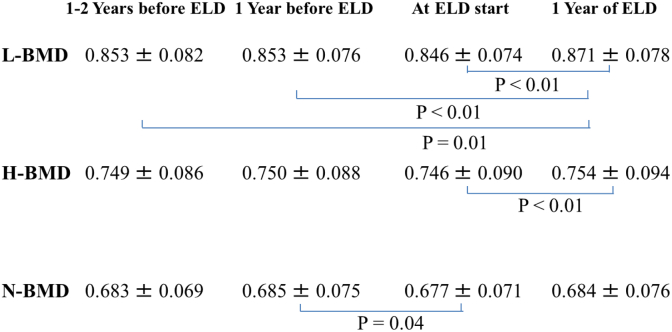
Fig. 3.
Value changes of bone mineral density (BMD) 1–2 years before eldecalcitol (ELD), 1 year before ELD, at ELD start, and 1 year of ELD. Lumbar BMD (L-BMD) tended to decrease prior to ELD. After 1 year of ELD treatment, L-BMD was 0.871 ± 0.078 g/cm2, which was a significant increase compared with values at any point before ELD addition (P< 0.01). Total hip BMD (H-BMD) also tended to decrease before ELD commencement. At 1 year of ELD addition, H-BMD was 0.754 ± 0.094 g/cm2 and significantly higher than that prior to ELD (P< 0.01). Femoral neck BMD (N-BMD) had similar a tendency to decrease prior to ELD. N-BMD was significantly decreased just before ELD treatment compared with at 1 year beforehand. At 1 year of ELD addition, N-BMD was 0.684 ± 0.076 g/cm2, which was not significantly higher than that prior to ELD. Values are presented as the mean ± standard deviation (n = 42).
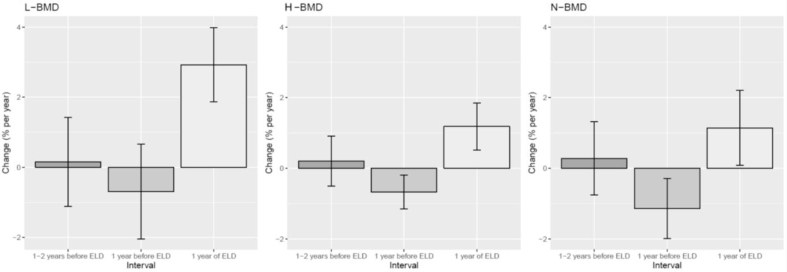
Fig. 4.
Percent changes in lumbar bone mineral density (L-BMD), total hip BMD (H-BMD), and femoral neck BMD (N-BMD) from 2 to 1 years before eldecalcitol (ELD), during the year before ELD, and during 1 year of ELD. For L-BMD, percent increases were 0.2% (95% confidence interval [95% CI] −1.1% to 1.4%, P= 0.81) from 2 to 1 years before ELD, −0.7% (95% CI, -2.0% to 0.7%, P= 0.30) during the year before ELD, and 2.9% (95% CI, 1.9% to 4.0%, P< 0.01) during 1 year of ELD administration. Regarding H-BMD, the respective percent increases were 0.2% (95% CI, -0.5% to 0.9%, P= 0.55), −0.7% (95% CI, -1.2% to −0.2%, P< 0.01), and 1.2% (95% CI, 0.5% to 1.9%, P< 0.01) for the time periods of 2 to 1 years before, the year before, and during 1 year of ELD administration. For N-BMD, percent increases were 0.3% (95% CI, -0.8% to 1.3%, P= 0.59) from 2 to 1 years before ELD, −1.1% (95% CI, -2.0% to −0.3%, P< 0.01) during the year before ELD, and 1.1% (95% CI, 0.1% to 2.2%, P= 0.03) during 1 year of ELD administration.
3.1. Bone formation marker
Prior to BP treatment, mean serum BAP was 19.5 ± 9.6 U/L, which had decreased significantly to 11.5 ± 3.6 U/L prior to ELD addition (P< 0.01). Afterwards, BAP further decreased significantly to 8.7 ± 2.2 U/L (P< 0.01) (Fig. 1).
3.2. Bone resorption marker
Before BP therapy, mean urinary NTX was 49.3 ± 25.9 nmol bone collagen equivalent (BCE)/mmol creatinine (Cr). This had decreased significantly to 25.0 ± 10.6 nmol BCE/mmol Cr prior to ELD (P< 0.01), and was further reduced after ELD addition by a significant degree to 17.0 ± 5.9 nmol BCE/mmol Cr (P= 0.01) (Fig. 2).
3.3. BMD results
L-BMD tended to decrease prior to ELD. After 1 year of ELD treatment, L-BMD was 0.871 ± 0.078 g/cm2, which was a significant increase compared with values at any point before ELD addition (P< 0.01) (Fig. 3). With respect to percent increases, these were 0.2% (95% confidence interval [95% CI] −1.1% to 1.4%, P= 0.81) from 2 to 1 years before ELD, −0.7% (95% CI, -2.0% to 0.7%, P= 0.30) during the year before ELD, and 2.9% (95% CI, 1.9% to 4.0%, P< 0.01) during 1 year of ELD administration. L-BMD increased significantly after starting ELD despite no observable gains beforehand (Fig. 4).
H-BMD also tended to decrease before ELD commencement. At 1 year of ELD addition, H-BMD was 0.754 ± 0.094 g/cm2 and significantly higher than that prior to ELD (P< 0.01) (Fig. 3). Respective percent increases were 0.2% (95% CI, -0.5% to 0.9%, P= 0.55), −0.7% (95% CI, -1.2% to −0.2%, P< 0.01), and 1.2% (95% CI, 0.5% to 1.9%, P< 0.01) for the time periods of 2 to 1 years before, the year before, and during 1 year of ELD administration. H-BMD increased significantly with ELD in spite of no prior gains (Fig. 4).
Femoral neck BMD (N-BMD) had similar a tendency to decrease prior to ELD. N-BMD was significantly decreased just before ELD treatment compared with at 1 year beforehand. At 1 year of ELD addition, N-BMD was 0.684 ± 0.076 g/cm2, which was not significantly higher than that prior to ELD (Fig. 3). The percent increases were 0.3% (95% CI, -0.8% to 1.3%, P= 0.59) from 2 to 1 years before ELD, −1.1% (95% CI, -2.0% to −0.3%, P< 0.01) during the year before ELD, and 1.1% (95% CI, 0.1% to 2.2%, P= 0.03) during 1 year of ELD administration. N-BMD increased significantly after ELD commencement despite no improvement beforehand (Fig. 4).
No adverse effects, such as fractures or hypercalcemia, were observed during the study period.
4. Discussion
In the present study of patients with primary OP, bone turnover markers were significantly decreased by BP administration and became further significantly suppressed by ELD addition. Although L-BMD, H-BMD, and N-BMD were no longer increased by chronic BP therapy, they exhibited significant increase rate gains following ELD commencement. Thus, ELD may be useful for osteoporotic patients exhibiting a diminished long-term BP therapy response.
ELD exerts inhibitory effects on bone metabolism [9]. In this study, BAP and urinary NTX were significantly reduced prior to BP therapy. They were further significantly suppressed after ELD addition, indicating that the drug significantly inhibited bone metabolism even under BP administration. A previous investigation revealed that BAP and tartrate-resistant acid phosphatase 5b were both significantly reduced by BPs after a regimen change from ALF to ELD [12]. In their comparison of a BP and ELD group with a BP and native vitamin D group, Sakai et al. witnessed that although urinary NTX was significantly suppressed by combined ELD therapy, the difference between the regimens was slight and without remarkable variation in BAP suppression [9], suggesting that the additive inhibitory effect on bone metabolism for combination ELD was modest. We also reported that in combined denosumab and ELD therapy, no significant changes were detectable among bone turnover markers [16]. Together, these results imply that ELD and antiresorption drugs may not sufficiently modulate bone metabolism when combined at the beginning of treatment. The precise mechanism of these observations is unknown and requires further study.
In a comparison of BPs with ELD or native vitamin D, Sakai et al. reported no significant differences in L-BMD or H-BMD increases, although N-BMD was significantly more increased by ELD [9]. Similar results were reported for BPs and denosumab [16], indicating that combination therapy of ELD and bone antiresorption drugs exerted effects that were limited to the femoral neck, which consists almost entirely of cortical bone. In this study, the addition of ELD to BP therapy produced significant percent gains in L-BMD, H-BMD, and N-BMD in BP poor-response patients. A previous investigation revealed that in BP treatment after a regimen change from ALF to ELD, L-BMD values increased significantly and N-BMD rose moderately with no gains in H-BMD [12], suggesting increased effects on BMD when ELD was added after long-term BP therapy rather than from the beginning. The reason for this discrepancy is unknown, and the mechanism of the increase in BMD caused by ELD addition in poorly responding patients apart from stronger inhibitory effects on bone resorption is unclear.
Randomized controlled trials are considered to provide the highest level of evidence. N-of-1 studies also yield superior analysis power when the subjects are the same, in which case statistical experts deem N-of-1 studies to be preferable [17,18]. N-of-1 studies necessitate a certain washout period and randomization. As the order of drug administration was not randomized, the current trial cannot be considered an N-of-1 study. However, the effectiveness of BPs on BMD also diminishes over long treatment periods (17,18); indeed, the effects of BPs on BMD in this investigation decreased over time, which was disadvantageous for ELD compared with no additional medication. Hence, the statistical meaning of our study may be considered as not inferior to that of an N-of-1 design.
This study demonstrated that BMD gains decreased substantially in BP poor-response cases over time. The addition of ELD increased both L-BMD and H-BMD, which indicated a possible benefit of this agent in long-term treatment regimes. The limitations of this study include a small sample size, short follow-up period, and no evaluation of fracture prevention during observation. As adequate levels of serum 25(OH)D3 are important in osteoporotic treatment, the lack of the data on serum 25(OH)D3 is another major shortcoming of this study. However, we recently observed that 3-year BP therapy without vitamin D supplementation significantly increased serum 25(OH)D3 [19]. Although not measured in this study, we presumed that serum 25(OH)D3 levels were sufficient in our patients owing to their prior long-term BP therapy. Future studies are required to confirm our findings.
5. Conclusion
In osteoporotic patients exhibiting a poor response to long-term BP therapy, ELD addition may further decrease bone turnover markers and increase L-BMD and H-BMD to represent a good treatment option.
Conflicts of interest
No potential conflict of interest relevant to this article was reported. ORCID. Mikio Kamimura: 0000-0003-1519-3047. Shota Ikegami: 0000-0001-6404-5249. Keijiro Mukaiyama: 0000-0002-8861-1679. Hidefumi Koiwai: 0000-0002-5358-7736. Yukio Nakamura: 0000-0002-3911-7180. Akira Taguchi: 0000-0003-2620-1487. Hiroyuki Kato: 0000-0002-4996-632X.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Lyles K.W., Colón-Emeric C.S., Magaziner J.S., Adachi J.D., Pieper C.F., Mautalen C. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaupre L.A., Morrish D.W., HanleyV D.A., Maksymowych W.P., Bell N.R., Juby A.G. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011;22:983–991. doi: 10.1007/s00198-010-1411-2. [DOI] [PubMed] [Google Scholar]
- 3.Hagino H., Shiraki M., Fukunaga M., Nakano T., Takaoka K., Ohashi Y. Three years of treatment with minodronate in patients with postmenopausal osteoporosis. J Bone Miner Metab. 2012;30:439–446. doi: 10.1007/s00774-011-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orimo H., Schacht E. The D-hormone analog alfacalcidol: the pioneer beyond the horizon of osteoporosis treatment. J Rheumatol Suppl. 2005;76:4–10. [PubMed] [Google Scholar]
- 5.Shiraki M., Kushida K., Fukunaga M., Kishimoto H., Taga M., Nakamura T. A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int. 1999;10:183–192. doi: 10.1007/s001980050214. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura Y., Suzuki T., Kamimura M., Ikegami S., Uchiyama S., Kato H. Alfacalcidol increases the therapeutic efficacy of ibandronate on bone mineral density in Japanese women with primary osteoporosis. Tohoku J Exp Med. 2017;241:319–326. doi: 10.1620/tjem.241.319. [DOI] [PubMed] [Google Scholar]
- 7.Hatakeyama S., Nagashima S., Imai N., Takahashi K., Ishihara J., Sugita A. Synthesis and biological evaluation of a 3-positon epimer of 1alpha,25-dihydroxy-2beta-(3-hydroxypropoxy)vitamin D3 (ED-71) J Steroid Biochem Mol Biol. 2007;103:222–226. doi: 10.1016/j.jsbmb.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto T., Ito M., Hayashi Y., Hirota T., Tanigawara Y., Sone T. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures–a randomized, active comparator, double-blind study. Bone. 2011;49:605–612. doi: 10.1016/j.bone.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Sakai A., Ito M., Tomomitsu T., Tsurukami H., Ikeda S., Fukuda F. Efficacy of combined treatment with alendronate (ALN) and eldecalcitol, a new active vitamin D analog, compared to that of concomitant ALN, vitamin D plus calcium treatment in Japanese patients with primary osteoporosis. Osteoporos Int. 2015;26:1193–2202. doi: 10.1007/s00198-014-2991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bone H.G., Hosking D., Devogelaer J.P., Tucci J.R., Emkey R.D., Tonino R.P. Treatment Study Group. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 11.Kamimura M., Nakamura Y., Ikegami S., Uchiyama S., Kato H., Taguchi A. Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate-unresponsive patients. Osteoporos Int. 2017;28:559–566. doi: 10.1007/s00198-016-3764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukaiyama K., Uchiyama S., Nakamura Y., Ikegami S., Taguchi A., Kamimura M. Eldecalcitol, in combination with bisphosphonate, is effective for treatment of Japanese osteoporotic patients. Tohoku J Exp Med. 2015;237:339–343. doi: 10.1620/tjem.237.339. [DOI] [PubMed] [Google Scholar]
- 13.Orimo H., Nakamura T., Hosoi T., Iki M., Uenishi K., Endo N. Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Arch Osteoporos. 2012;7:3–20. doi: 10.1007/s11657-012-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikegami S., Kamimura M., Uchiyama S., Mukaiyama K., Kato H. Unilateral vs bilateral hip bone mineral density measurement for the diagnosis of osteoporosis. J Clin Densitom. 2014;17:84–90. doi: 10.1016/j.jocd.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Ikegami S., Kamimura M., Uchiyama S., Nakamura Y., Mukaiyama K., Kato H. Clinical implications of hip flexion in the measurement of spinal bone mineral density. J Clin Densitom. 2016;19:270–276. doi: 10.1016/j.jocd.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T., Nakamura Y., Tanaka M., Kamimura M., Ikegami S., Uchiyama S. Comparison of the effects of denosumab with either active vitamin D or native vitamin D on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Mod Rheumatol. 2018;28:376–379. doi: 10.1080/14397595.2017.1308454. [DOI] [PubMed] [Google Scholar]
- 17.Cook D.J., Guyatt G.H., Davis C., Willan A., McIlroy W. A diagnostic and therapeutic N-of-1 randomized trial. Can J Psychiatry. 1993;38:251–254. doi: 10.1177/070674379303800405. [DOI] [PubMed] [Google Scholar]
- 18.Gabler N.B., Duan N., Vohra S., Kravitz R.L. N-of-1 trials in the medical literature: a systematic review. Med Care. 2011;49:761–768. doi: 10.1097/MLR.0b013e318215d90d. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y., Uchiyama S., Kamimura M., Ikegami S., Komatsu M., Kato H. Increased serum 25(OH)D3 levels in post-menopausal Japanese women with osteoporosis after 3-year bisphosphonate treatment. Tohoku J Exp Med. 2017;242:241–246. doi: 10.1620/tjem.242.241. [DOI] [PubMed] [Google Scholar]