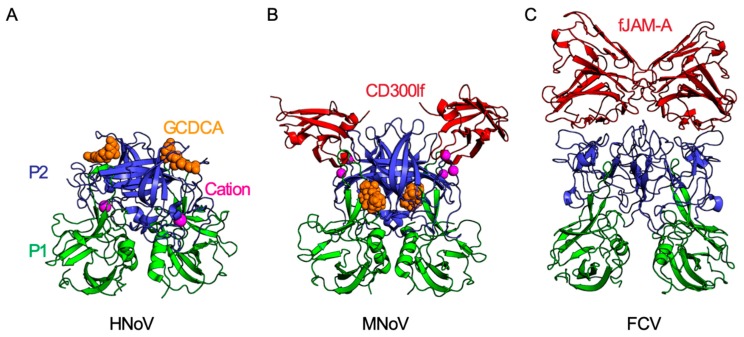
Figure 2.
Molecular interactions of calicivirus capsid proteins. Each calicivirus has T = 3 icosahedral symmetry and is comprised of 90 VP1 dimers. Each VP1 capsid monomer is comprised of a shell (S) and a protruding (P) domain. The P domain is further subdivided into an apical P2 domain (blue) and a P1 domain (green) which connects the more variable P2 to the shell domain. (A) The secondary bile salt glycochenodeoxycholic acid (GCDCA; shown as orange space-filling model) binds to the apical region of the P2 domain of human norovirus (HNoV) GII.10 (PDB ID: 6GW1) [38]. This binding site partially overlaps with the HBGA binding site on HNoVs (not shown). (B) MNoV P2 binds the CD300lf receptor (red) at 1:1 stoichiometry (PDB IB: 6E47). Both cations (magenta) and GCDCA (orange) enhance binding of MNoV to CD300lf. GCDCA binds MNoV VP1 at the dimer interface between the protruding domains, a site different from the GCDCA-binding site on HNoV GII.10 [25,26,27]. (C) The apical region of FCV capsid P2 domain binds the FCV receptor fJAM-A (PDB ID: 6GSI). Similar to CD300lf, fJAM-A is an Ig domain-containing membrane protein and binds the P2 domain at 1:1 stoichiometry. While the CD300lf ectodomain is comprised of a single Ig domain, fJAM-A has two Ig domains (D1 and D2) with only the distal D1 domain directly binding the FCV P2 domain [34].