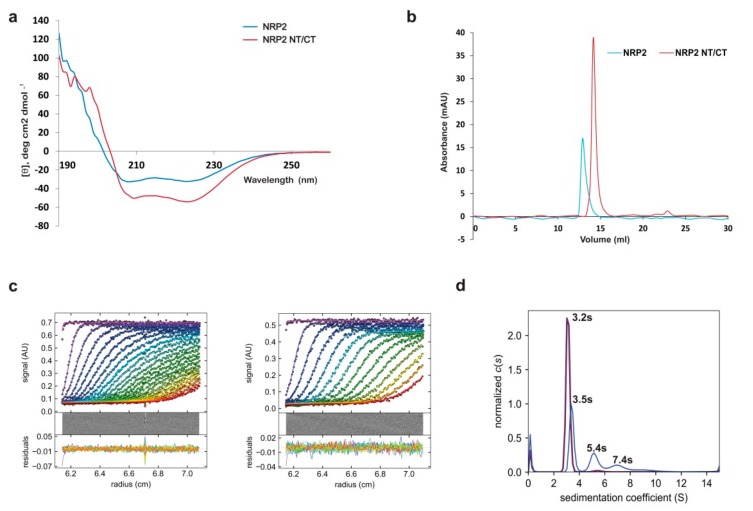
Figure 3.
Secondary structure and oligomerization status determination of AtNRP2. (a) The near-UV Circular dichroism (CD) spectroscopy profile of full-length AtNRP2 and AtNRP2 NT/CT, showing them to be predominantly α-helical in composition. (b) Analytical gel-filtration chromatogram of full-length AtNRP2 and AtNRP2 NT/CT. The elution volumes obtained were nearly 12.9 and 14.0 mL, respectively, which correspond to the sizes bigger than those of the dimers. (c) Sedimentation velocity analytical ultra-centrifugation of AtNRP2 (left panel) and AtNRP2 NT/CT (middle panel). (d) The plot shows the fitting on c(s) analysis and the distribution of sedimentation coefficients for both the proteins respectively (right panel). Peaks in blue represent full-length AtNRP2 and peaks in purple represent AtNRP2 NT/CT. The higher ‘s’ value corresponds to bigger molecules.