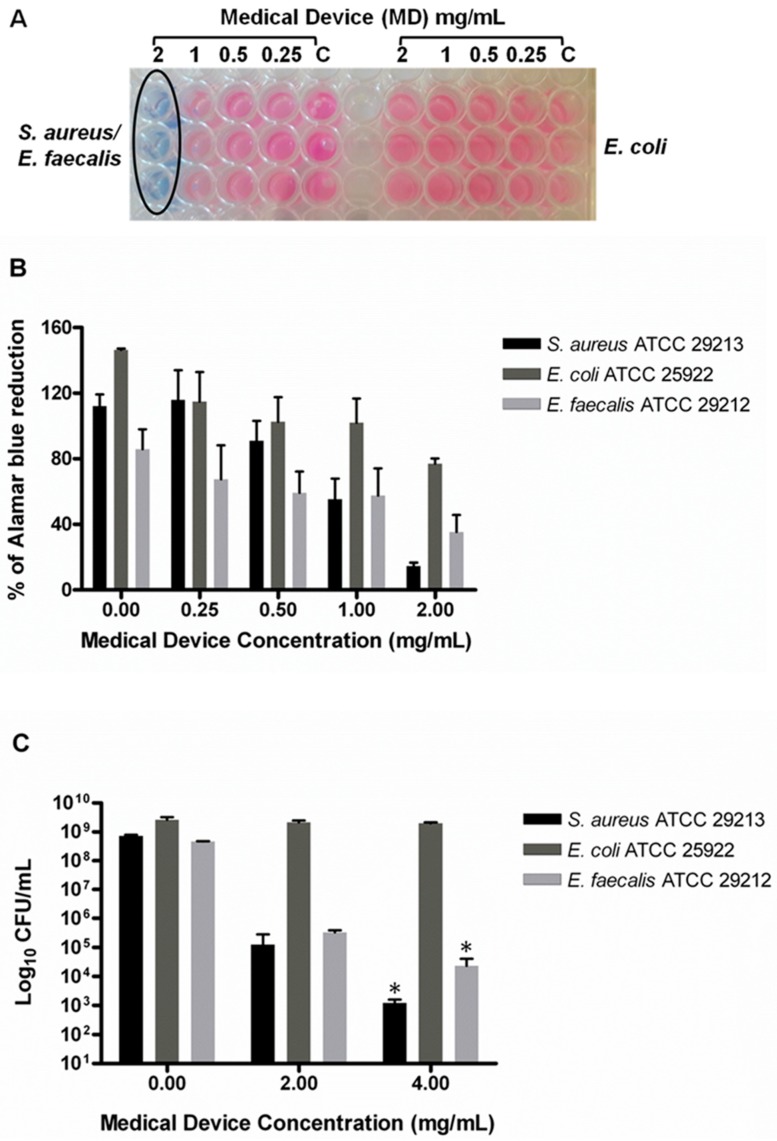
Figure 1.
Evaluation of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the medical device (MD) versus S. aureus, E. coli, and E. faecalis. The MIC was determined by Alamar Blue® (AB) assay, which is based on the chemical reduction of resazurin (a non-toxic, cell-permeable, non-fluorescent, blue compound) in resorufin, which is a highly fluorescent red/purple compound. Such reduction is performed by viable cells; therefore, continued cell growth maintains a reduced environment, while the inhibition of growth or cell death induces an oxidized environment. (A) Representative image of colorimetric MIC determination using AB assay. The black circle indicates the MIC at 2 mg/mL which is the same for both S. aureus and E. faecalis. (B) The plot shows the percentage reduction of AB recorded for the three bacterial species at different MD concentrations compared to the corresponding untreated samples (0.00) performed as described in the Materials and Methods section. The percentage of AB reduction was evaluated by using absorbance at 570 nm and 600 nm. (C) The MBC was determined by CFU counts and corresponds to 4 mg/mL for S. aureus and E. faecalis. Data are the mean of three replicates of three independent experiments; * p < 0.05 vs. the controls (0.00). C: controls or untreated samples.