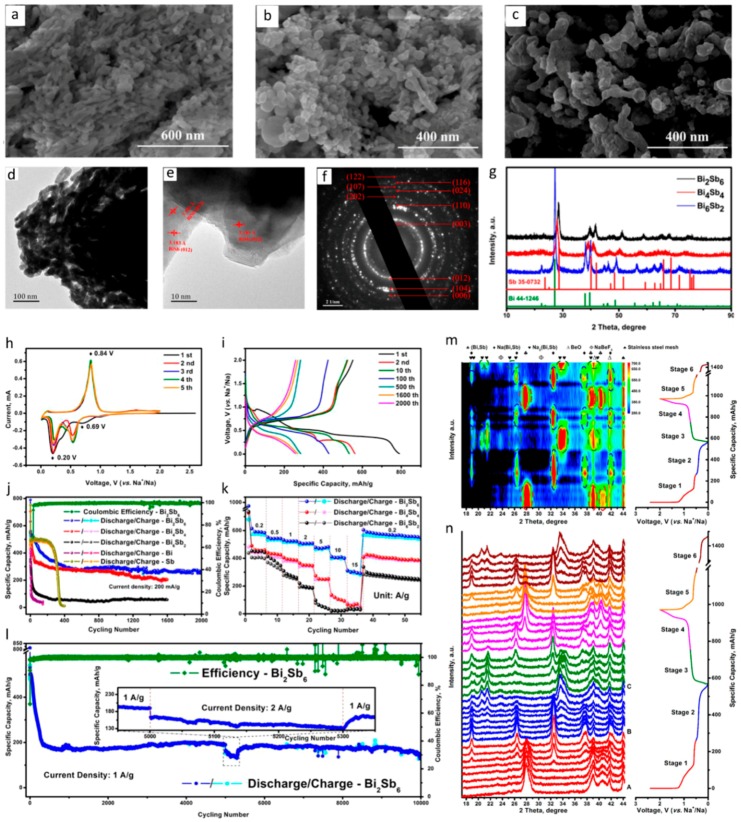
Figure 5.
Structural characterization and electrochemical performance analysis of intermetallic alloy (np-Bi-Sb) as an anode in SIBs: (a–c) SEM images of the np-Bi2Sb6, np-Bi4Sb4, and np-Bi6Sb2 samples, respectively showing typical three-dimensional bi-continuous ligament-channel with various size of ligaments. (d) TEM image of the np-Bi4Sb4 features the nanoporous structure of np-Bi-Sb alloys. (e) The high resolution TEM (HRTEM) image shows that the crystal size (~25 nm) of the np-Bi-Sb alloys are approximately close to the ligament’s sizes. (f) The selected-area electron diffraction (SAED) pattern of the np-Bi-Sb alloy further confirms the nanocrystalline structure of the selected region. (g) XRD patterns of the np-Bi-Sb alloys are indexed to the single phase of BiSb alloy. (h) Cyclic voltammograms (CV) of the np-Bi2Sb6 electrode at a scan rate of 0.1 mV s−1 over a potential window of 0.01−2.0 V (vs. Na+/Na). (i) GCD curves of the np-Bi2Sb6 electrode in different cycles at a current density of 200 mA/g. The overlap of the charge/discharge curves of the 1600th and 2000th cycles implies the excellent stability during the long cycling. (j,k) Excellent cycling performance and rate capability of the np-Bi-Sb alloys, respectively. (l) Cycling performance and coulombic efficiency of the np-Bi2Sb6 electrode at 1 A g−1 exhibit amazing cycling capability. A zoom-in image (inset) illustrates the cycling performance at 2 A g−1 when the discharge capacity can remain about 150 mAh g−1 from 5000 to 5300 cycles. (m–n) Contour plot and line plot of the operando XRD of the np-Bi-Sb alloys during the charge/discharge processes reveals the sodiation/desodiation procedures of the alloy as the anode of the Na-ion batteries. Reprinted with permission from [102]. Copyright 2018 American Chemical Society.