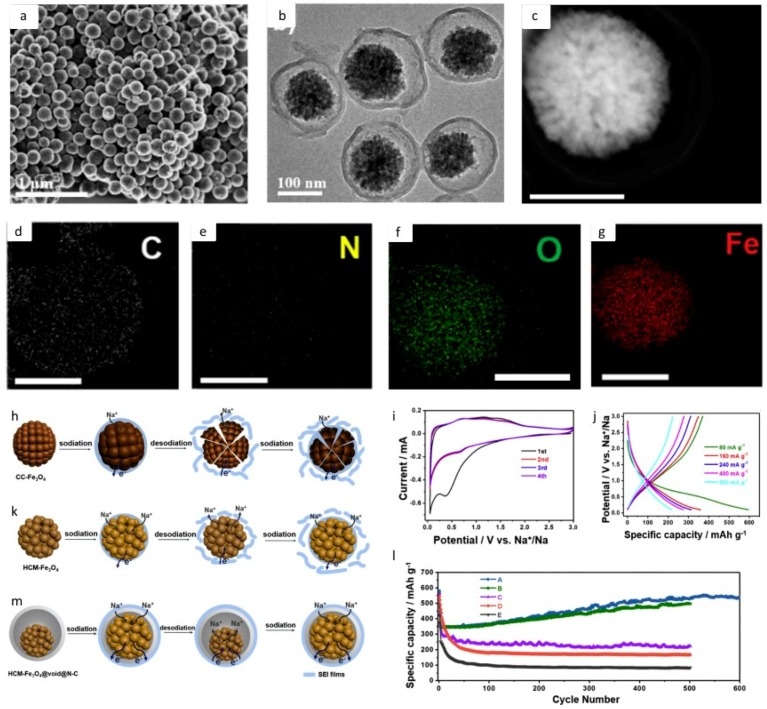
Figure 6.
Structural characterization and electrochemical performance analysis of metal oxide yolk-shell structured HCM-Fe3O4@void@N–C nanospheres as an anode in SIBs: (a) field emission scanning electron microscope (FESEM) of the material, (b) transmission electron microscopy (TEM) of the material, (c) scanning transmission electron microscopy (STEM) of the material, (d–g) energy dispersive X-ray (EDX) elemental mappings of a HCM-Fe3O4@void@N–C nanosphere, (h) representation of the sodiation and desodiation processes of CC-Fe3O4 and their characteristic volume increases, (i) cyclic voltammetry curves from 1st to 4th cycles for voltage range from 0.01–3.0 V. It is worth noting that the first cycle shape differs from the other cycles because of side reactions and Solid Electrolyte Interphase (SEI) formation, (j) charge–discharge curves for different current densities of HCM-Fe3O4@void@N–C nanospheres, (k) representation of the sodiation and desodiation processes of HCM-Fe3O4, (l) specific capacity representation versus cycle number for current density of 160 mAg−1 of the following materials: (A) HCM-Fe3O4@void@N–C, (B) HCM-Fe3O4@void@C, (C) HCM-Fe3O4@C, (D) HCM-Fe3O4, and (E) CC-Fe3O4 nanospheres, and (m) representation of the sodiation and desodiation processes of HCM-Fe3O4@void@N–C nanospheres, showing the SEI film, which accommodates the volume expansion characteristic of Fe3O4 species. The bars of (c–g) are 100 nm. Reprinted with permission from [51]. Copyright 2019 Elsevier B.V. All rights reserved.