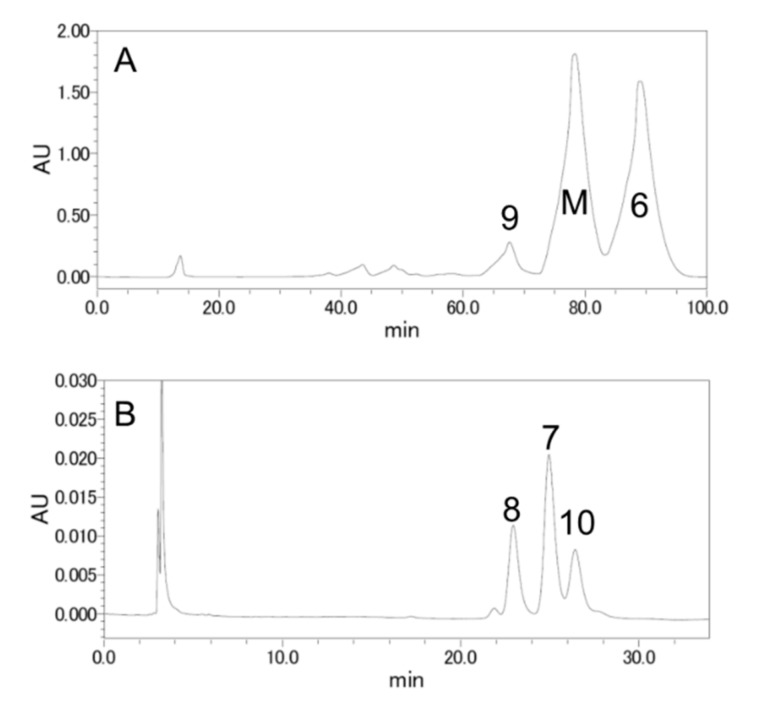
Figure 3.
HPLC profile of target compounds 6–9 and compound 10. After final reaction with 0.1% BHA in cyclohexane under reflux conditions for 1 h, the reaction mixture was concentrated. The compound which has the same Rf value (Rf = 0.4, AcOEt/n-Hex = 3:7) of TLC was purified using silica-gel column chromatography (AcOEt/n-Hex = 1:4). This compound was further purified with reverse-phase HPLC column chromatography, and the profile was shown in (A) (column: Mightysil RP-18 GP 250-20 (5 μm) Kanto Chemical Co., Inc, solvent: 100% acetonitrile; initially the flow rate was at 1 mL/min and it was increased to 5 mL/min over 1 min, and this condition was maintained, detector: 254 nm absorbance). As a result of NMR analyses, peak 9 (67.6 min) was attributed to compound 9 (vitamin D2), and peak 6 (88.9 min) was attributed to compound 6 (vitamin D5). Unfortunately, peak M (78.4 min) was a mixture. Peak M was further purified using a special HPLC column, and the profile was shown in (B) (column: COSMOSIL Cholester 4.6 × 250 mm Nacalai Tesque Inc, solvent: 100% acetonitrile; 1 mL/min, detector: 254 nm absorbance). As a result of NMR analyses, peak 8 (22.9 min) was attributed to compound 8 (vitamin D6), peak 7 (24.9 min) was attributed to compound 7 (vitamin D7), and peak 10 (26.4 min) was attributed to vitamin D4 (Figure 4. Compound 10).