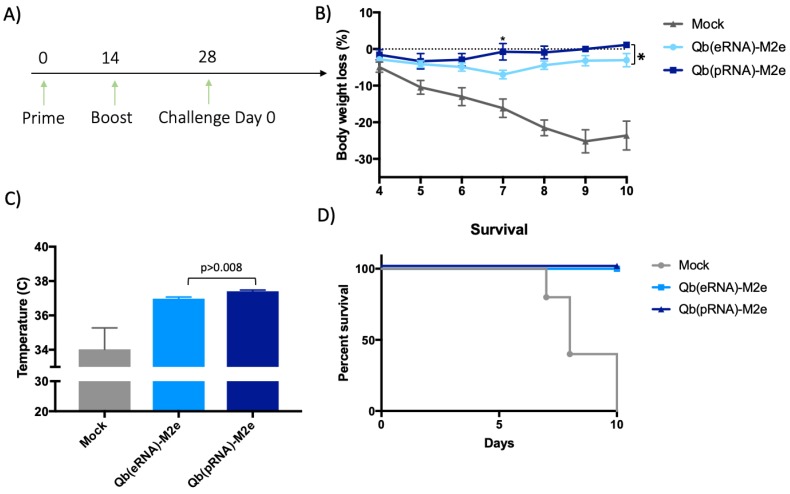
Figure 5.
Reduced morbidity symptoms of mice immunised with formulations containing prokaryotic RNA in an influenza challenge. (A) Immunisation and challenge schedule. 8–12 weeks female BALB/c mice were immunised s.c. with 50 μg of Qβ(pRNA)-M2e or Qβ(eRNA)-M2e, or PBS as a control on day 0 and 14. Mice were then challenged with a lethal dose of the mouse-adapted influenza A/PR/8/34 virus (2 × LD50) on day 28. (B) Body weight (%) represented as the mean +/− SEM percentage of weight loss (n = 5 mice). Statistical significance measured by Welch’s T test (p < 0.007). (C) Average temperature from day 4 to 11 post challenge. mean +/− SEM (n = 5 mice). Statistical significance measured by Welch’s T test (p < 0.008). (D) Percentage of survival of immunised and control groups following challenge. *: One mouse from the Qβ(pRNA) group was prematurely culled due to unrelated sickness. Mice that reached the limit severity score were euthanised and the last observed parameter was used for the following days and average calculations (Last observation carried forward, LOCF).