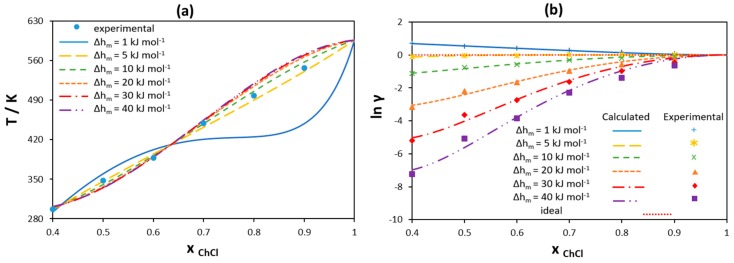
Figure 8.
(a) Liquidus line of [Ch]Cl in a [Ch]Cl/choline acetate binary mixture modeled using the RK-polynomial assuming different melting enthalpy values. (b) Activity coefficients of [Ch]Cl in the liquid phase calculated using the RK-polynomial assuming different melting enthalpy values. Melting temperature of [Ch]Cl is assumed to be 597 K. Experimental data are taken from [56].