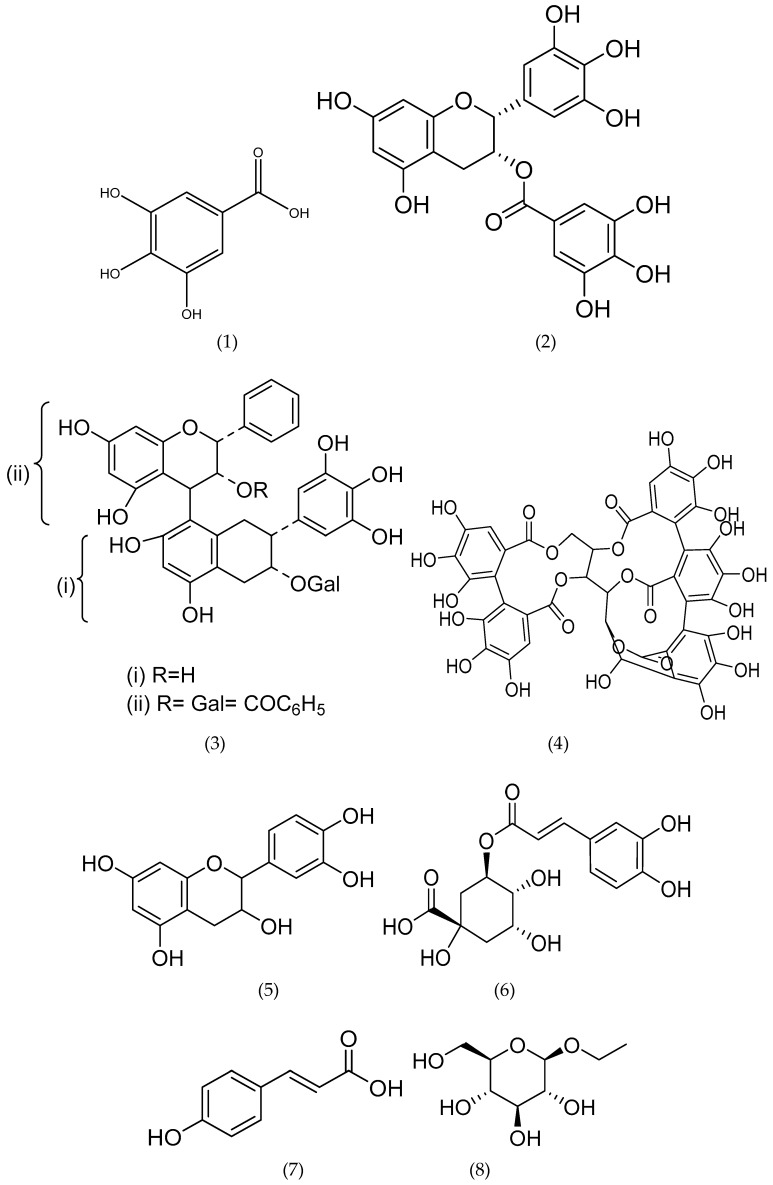
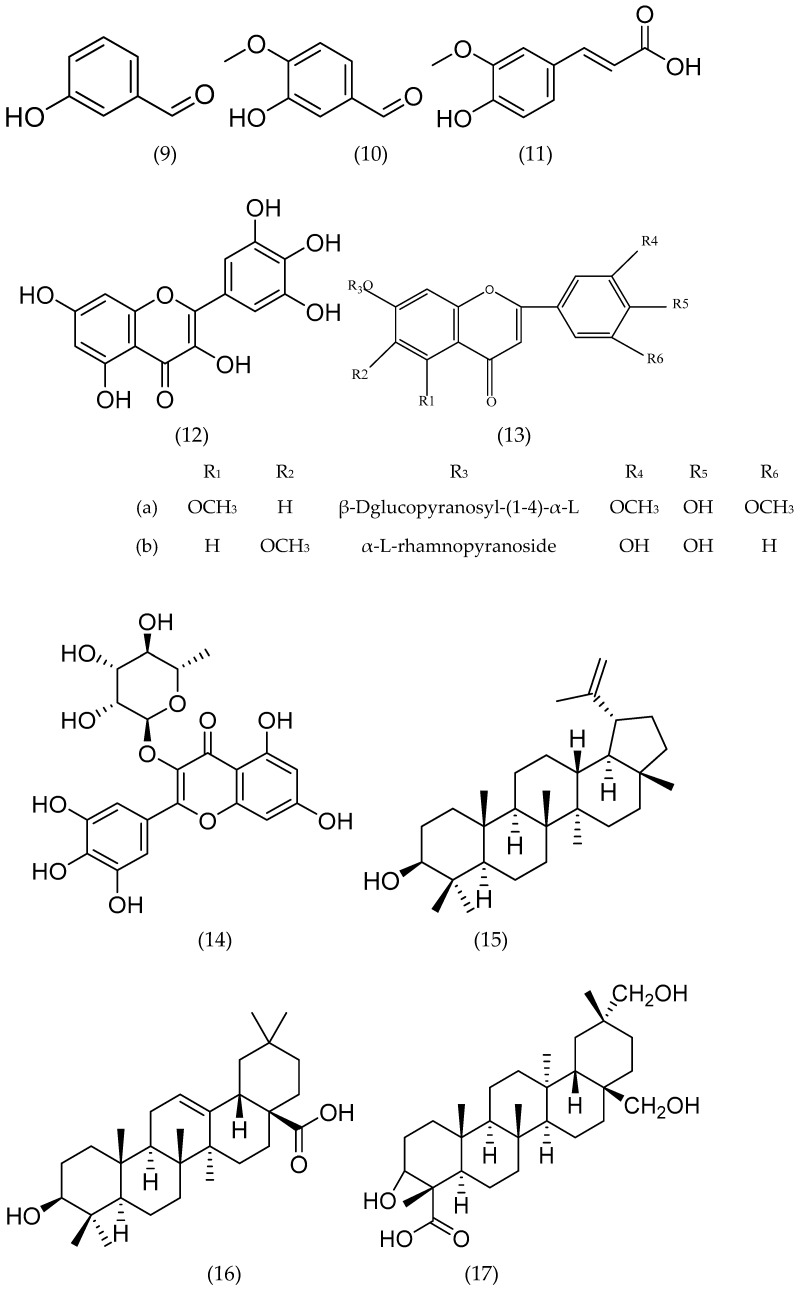
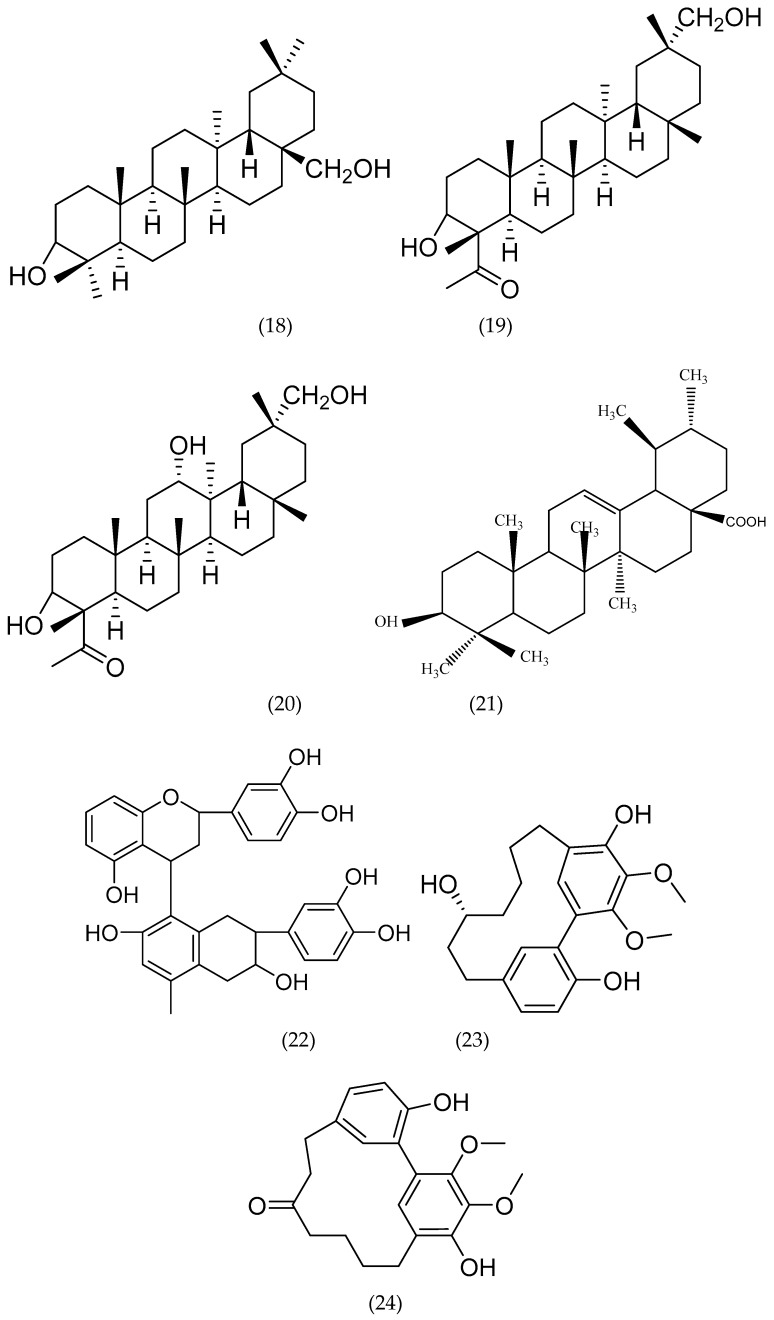
Figure 4.
Structure of some isolated bioactive compounds from different parts of M. esculenta. (1) Gallic acid, (2) Epigallocatechin 3-O-gallate, (3) i) Epigallocatechin-(4β→8)-epigallocatechin-3-O-gallate, ii) 3-O-galloyl-epigallocatechin-(4β→8)-epigallocatechin-3-O-gallate, (4) Castalagin, (5) Catechin, (6) Chlorogenic acid, (7) p-coumaric acid, (8) Ethyl-β-D-glucopyranoside, (9) 3-hydroxybenzaldehyde, (10) Isovanillin, (11) Ferulic acid, (12) Myricetin, (13) i) Flavone 4′-hydroxy-3′,5,5′-trimethoxy-7-O-β-D-glucopyranosyl(1→4)-α-L-rhamnopyranoside, ii) flavone 3′,4′-dihydroxy-6-methoxy-7-O-α-L-rhamnopyranoside, (14) Myricitrin, (15) Lupeol, (16) Oleanolic acid, (17) Trihydroxytaraxaranoic acid, (18) Dihydroxytaraxerane, (19) Dihydroxytaraxaranoic acid, (20) Tetrahydroxytaraxenoic acid, (21) 3-epi-ursonic acid, (22) Prodelphinidin dimer, (23) Myricanol, (24) Myricanone.