Abstract
Cannabinoids are the most commonly abused illicit drugs worldwide. While cannabis can be beneficial for certain heath conditions, abuse of potent synthetic cannabinoids has been on the rise. Exposure to cannabinoids is also prevalent in women of child-bearing age and pregnant women. These compounds can cross the placental barrier and directly affect the fetus. They mediate their effects primarily through G-protein coupled cannabinoid receptors, CB1 and CB2. In addition to significant neurological effects, cannabinoids can trigger robust immunomodulation by altering cytokine levels, causing apoptosis of lymphoid cells and inducing suppressor cells of the immune system. Profound effects of cannabinoids on the immune system as discussed in this review, suggest that maternal exposure during pregnancy could lead to dysregulation of innate and adaptive immune system of developing fetus and offspring potentially leading to weakening of immune defenses against infections and cancer later in life. Emerging evidence also indicates the underlying role of epigenetic mechanisms causing long-lasting impact following cannabinoid exposure in utero.
Keywords: Fetus, Immune system, Marijuana, Metabolites, Neurological, Pregnancy, Perinatal, Prenatal, Substance abuse
Introduction
Cannabis and synthetic cannabinoids are considered one of the most common drugs of abuse [1–3]. There has also been an intense public interest with regard to their health benefits and a greater acceptance of medical cannabis in recent years [4, 5]. The current decriminalization and legalization efforts for the recreational use of cannabis, as well as a renewed interest in its therapeutic use are expected to lead to an increase in the prevalence of exposure to these drugs in the coming years [6, 7]. These call for a clear understanding of risks of cannabinoid exposure during pregnancy. Historically, the constellation of effects of cannabinoid use during pregnancy has not received enough attention. While the early and long-term developmental and neurological adverse effects of prenatal cannabis abuse have been known for some time, the profound immunological implications are only beginning to emerge in recent literature. In this review, we discuss the prevalence and recent trends of abuse of cannabis and synthetic cannabinoids during pregnancy and address their impact on the endocannabinoid system (ECS) and immune function of the developing fetus and offspring. We will discuss the possible underlying mechanisms involving cytokines and cells of the immune system. Further, we will highlight the emerging role of epigenetic mechanisms of immune dysregulation caused by maternal exposure to cannabinoids during pregnancy.
Cannabis and synthetic cannabinoids
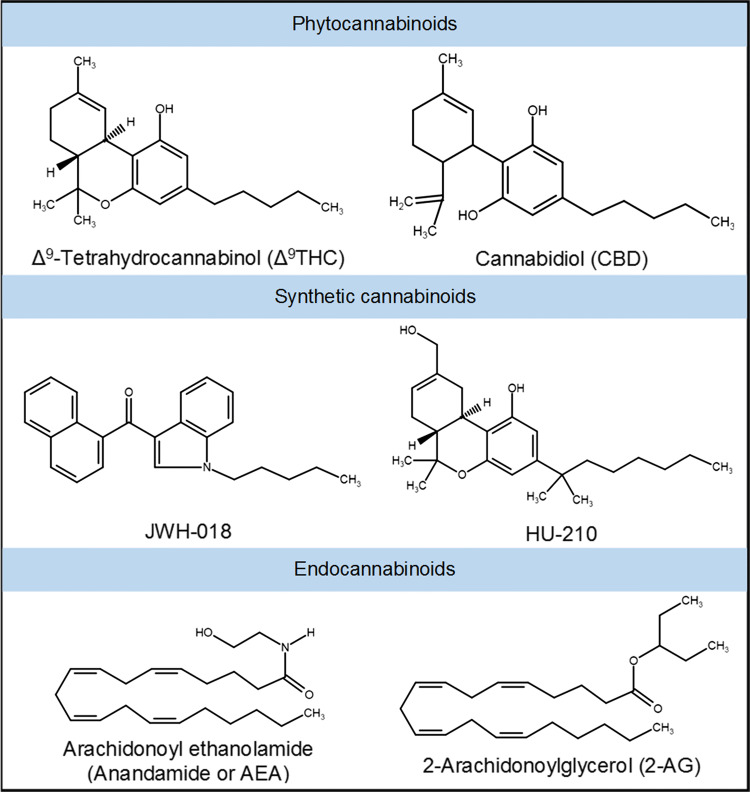
Cannabis (or marijuana) refers to the dried leaves, flowers, stems, and seeds from the plant Cannabis sativa, which contains the major psychoactive chemical delta-9-tetrahydrocannabinol (Δ9THC) as well as other related compounds collectively called phytocannabinoids (Fig. 1) [8–10]. It has been used for centuries not only for recreational purposes but also for its actual as well as perceived medicinal benefits [11]. It is believed to be beneficial in symptomatic relief of a variety of ailments. For instance, the use of cannabis and cannabis-derived cannabidiol (CBD) preparations for effective alleviation of seizers in children with epilepsy who do not respond to other medications is well known [12]. Cannabis and Δ9THC are also effective in providing relief from nausea, vomiting and loss of appetite in cancer patients undergoing chemotherapy as well as in AIDS patients [13]. In fact, the synthetic Δ9THC such as Dronabinol (Marinol™, Syndros™) and Nabilone (Cesamet™) are FDA approved as antiemetics and orexigenic drugs for this purpose. A mixture of Δ9THC and CBD (Sativex®) is approved for medical use in Europe and Canada for treating spasticity and neuropathic pain in multiple sclerosis (MS) patients [13]. Potent activity of cannabinoids in reducing inflammation has also been demonstrated in various preclinical models [14], and there is an interest in developing cannabimimetics as novel anti-inflammatory therapeutics.
Fig. 1.
Chemical structures of major cannabinoids. Phytocannabinoids are natural cannabinoids derived from Cannabis plant. Synthetic cannabinoids are potent cannabimimetics commonly present in designer street drugs such as K2 or Spice. Cannabinoid-like compounds endogenously produced in humans and other animals are referred to as endocannabinoids. Most cannabinoids typically signal through cannabinoid receptors, CB1 and/or CB2
Natural cannabis and a number of cannabinoid compounds including Δ9THC are classified as Schedule I substances based on the United States federal Controlled Substances Act. It is illegal to cultivate, possess, trade and consume cannabis in majority of the countries worldwide, although the extent of implementation of such laws, and hence the prevalence of its use may vary widely. Nevertheless, it is considered the most widely cultivated and trafficked drug of abuse. According to the World Health Organization, cannabis is consumed by ~ 147 million people or nearly 2.5% of the global population [15]. In the United States, cannabis is the third most widely abused drug by adults, next to alcohol and tobacco. However, recent surveys since 2010 have also found that adolescents smoked cannabis more than cigarettes [16]. Approximately 46% of young adults (ages of 18–34 years) have used cannabis in their lifetime; and 2–3% of the population consumes cannabis on a daily basis [17]. The prevalence of cannabis use has also been increasing among youth and teens since 2007 [16, 18–20]. There have been increasing efforts in recent years towards easing restrictions on both medical and recreational use of cannabis. Several states in America have legalized it for recreational use, and 28 states have now passed laws allowing medical cannabis for certain health conditions [21]. With more states expected to join this trend, the cannabis use and abuse are anticipated to increase. Moreover, the amount of Δ9THC in cannabis has increased over the past decades, because of selective breeding practices for higher psychoactive content. Compared to approximately 4% in 1980s, Δ9THC concentrations in new cultivars of cannabis tremendously increased to about 15% in 2012 [22].
An alarming rise in the availability and abuse of highly potent synthetic cannabinoids has been considered as a public health emergency, with increasing number of overdoses and emergency room visits in large metropolitan areas [19, 23, 24]. Synthetic cannabinoids are mind-altering chemicals or mixtures of chemicals structurally related to Δ9THC that are sprayed on dried and shredded plant material, and sold. They are known by street names such as fake weed, K2 or Spice, often wrongly promoted as legal cannabis. Synthetic cannabinoid analogs such as 1-pentyl-3-(1-naphthoyl)indole (JWH-018), 1-butyl-3-(1-naphthoyl)indole (JWH-073) and 1,1-dimethylheptyl-11-hydroxytetrahydrocannabinol (HU-210) (Fig. 1) are some of the most commonly found chemicals in these products [25, 26]. They are also sold as herbal or liquid incense to be vaporized and inhaled in e-cigarettes and other devices. Their abuse trend has also been associated with increasing popularity of e-cigarettes and vapes among teens and younger population [19]. Synthetic cannabinoids are much more powerful than cannabis or Δ9THC, sometimes over 100 times stronger with potent psychoactivity and likely, with a myriad of other known and unknown adverse health effects on human body [27–29]. For example, HU-210 is a remarkably potent synthetic agonist which has a high affinity for both CB1 (Ki 0.0608 nM) and CB2 (Ki 0.524 nM) receptors [30]. It also exhibits high relative intrinsic activities at these receptors. Moreover, HU-210 is known to exhibit long half-life and prolonged duration of action in vivo. The high affinity and efficacy at cannabinoid receptors have been mainly attributed to the replacement of the pentyl side chain of THC with a dimethylheptyl group in HU-210 [30, 31].
Cannabinoid abuse during pregnancy
Cannabis is the most widely used illicit drug among women of childbearing age. In recent years, cannabis use appeared to increase among women in their reproductive years. In one of the Monitoring the Future Studies by NIDA, 10.4% of women aged 19–32 years reported using cannabis [32]. A recent survey has documented that approximately 4.9% of women of childbearing age regularly smoke cannabis [33]. The prevalence of substance abuse during pregnancy may vary from 5 to 16% [34]. It is estimated that five million women of childbearing age use illicit drugs and that approximately half a million infants in the United States are exposed to one or more illicit drugs in utero. Hence, the impact of maternal substance abuse on both the mother and offspring is of major public health concern. Based on National Pregnancy and Health Survey conducted by National Institute on Drug Abuse (NIDA), the prevalence and substance use patterns among women delivering live-born infants in the US, the self-reported cannabis use during pregnancy was 2.9% compared with 0.9–1.1% for cocaine [35, 36]. While the proportion of substance abuse treatment admissions for pregnant women in the United States remained stable at 4% during 1992–2012, those pregnant women reporting cannabis use increased significantly from 29 to 43% [37]. These studies have also found that pregnant women who use illicit drugs are more likely to use cannabis compared to other substances. This is often due to the perception that cannabis may be less harmful to the developing embryo and fetus compared to other drugs such as cocaine, heroin, or methamphetamine. With the legalization and decriminalization of medical and recreational cannabis in several states, its use among women and during pregnancy is expected to further increase in the coming years [38].
Accumulating evidence suggests that cannabis exposure during pregnancy may significantly impact fetal brain development causing neurological impairments, hyperactivity, poor cognitive function and changes in dopaminergic receptors in children [35, 39–44]. Notably, recent experimental evidence in rodents point to significant impact of stimulation of cannabinoid system on learning and reward-related explicit memory [45, 46]. While cannabis use during pregnancy did not increase the risk of perinatal mortality, regular use of cannabis throughout pregnancy was associated with a significant decrease in birth weight [35, 47]. However, a recent study found that cannabis use, after adjusting for tobacco and other illicit drug use, was associated with neonatal morbidity or death [48].
Endocannabinoid system (ECS) and pregnancy
The effects of cannabis on reproductive and immune functions may be closely related to the processes that are modulated by the ECS. Endogenous cannabinoids (endocannabinoids or eCBs) together with cannabinoid receptors, metabolic enzymes and membrane transporters form the ECS [49, 50]. Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the two major endocannabinoids (Fig. 1) [51, 52]. AEA is metabolized by fatty acid amide hydrolase (FAAH) to arachidonic acid (AA) and ethanolamine, while 2-AG is mainly degraded by monoacyl glycerol lipase (MAGL) to AA and glycerol [53, 54]. Although the precise mechanisms are not fully known, it is agreed that ECS plays a pivotal role in reproduction [55, 56]. The components of ECS are involved in fertilization, oviduct transport, implantation, embryo development, maintenance and immune regulation during pregnancy [57–59].
FAAH activity has been linked to early pregnancy success with a strong correlation between its decrease in maternal peripheral blood mononuclear cells and spontaneous miscarriage in women [60]. The levels and activity of FAAH were significantly lower in patients undergoing embryo transfer following in vitro fertilization who failed to achieve successful implantation as compared to those who became pregnant [61]. FAAH is considered as a critical metabolic gatekeeper of AEA levels in the uterus throughout the menstrual cycle, as well as during pregnancy [62, 63]. In a major study, uterine AEA levels were shown to be highest during the non-receptive stage and in inter-implantation sites, but lowest at the site of embryo implantation [64]. Thus, downregulation of AEA is associated with uterine receptivity, and its elevated levels with uterine refractoriness to embryo implantation. This suggests that the down-modulation of uterine AEA at the implantation sites may be the mechanism by which embryos protect themselves from detrimental effects of this endocannabinoid [64]. AEA also modulates decidualization of rat and human endometrial stromal cells [65, 66] and sperm functions required for fertilization in human reproductive tracts [67]. Deregulation of metabolic enzymes of endocannabinoids following exposure to cannabis has been, therefore, implicated in potential negative impacts on human fertility [65]. In fact, Δ9THC is known to significantly influence bioactive lipid profile or induce endocannabinoid levels [68, 69]. Unlike endocannabinoids, Δ9THC is metabolized slowly and may mimic situations in which an excess of endocannabinoids are produced or when re-uptake or removal of endocannabinoids is impaired [58]. Further, THC and CBD can have non-CB1/CB2 targets which may produce a more complex signaling cascade and additional implications for pregnancy.
Several studies have explored the role of intricate endocannabinoids-sex hormone-cytokine regulatory axis during pregnancy [57, 70]. Sex steroid hormones, progesterone and estrogen, are involved in the maintenance of endocannabinoid levels [71]. Progesterone promotes lymphocyte FAAH activity involving transcription factors Stat3 and Ikaros, which results in lower AEA levels [72–74]. These studies indicate regulation of immune cytokine network by endocannabinoids during reproduction which appears to be one of the important mechanisms controlling implantation and the maintenance of healthy pregnancy [75]. Animal studies have shown the presence of constituents of ECS in early embryo before neurogenesis indicating its involvement in early embryogenesis [76–78]. Thus, maternal abuse of cannabis and synthetic cannabinoids can cause adverse reproductive, developmental and immune consequences also by significantly altering the components of the ECS.
Pregnancy and immunity
The maternal immune system actively tolerates the semiallogeneic fetus during pregnancy. This includes changes in local immune responses in the uterine mucosa as well as alterations in peripheral immune responses [79, 80]. The innate immune system is activated during pregnancy [81, 82]. Cells of the granulomonocytic lineage significantly increase during normal pregnancy and undergo phenotypic and functional activation [83], whereas the dendritic cell numbers decrease [84]. Further, the number of natural killer (NK) T cells and the production of interferon (IFN)-γ by NK cells is decreased in pregnant women [85]. Pregnant women are more sensitive to certain infections and immune dysregulation caused by either proinflammatory or immunosuppressive stimuli. Thus, a significantly altered immune system is essential during pregnancy for normal placentation and maintenance of a healthy pregnancy. However, interfering with the maternal immune system could disturb the balance between tolerance and immunity during pregnancy and may affect the outcome.
Prenatal cannabinoid exposure and immune dysregulation
Exogenous cannabinoids, such as Δ9THC, have been shown to cross the fetal–placental barrier in humans and other mammals [86–89]. Significant effects of prenatal, intrauterine exposure to cannabinoids on the growth and development of the fetus, as well as on learning and memory, neuronal, behavioral and endocrine aspects of the progeny have been studied and reviewed [43, 90–92]. In addition to its effects on the central nervous system (CNS), cannabinoids also profoundly alter immune function [93–96]. While CB1 receptor is expressed in brain and CB2 in the peripheral tissues, immune cells express both the receptors [97–99]. Moreover, reproductive tissues such as the uterine endometrium, human placenta and ovaries express functional cannabinoid receptors [100–103]. However, only a few studies have investigated its impact on maternal and developing immune systems under normal or disease conditions. These studies as discussed below suggest that maternal Δ9THC exposure may have long-lasting effects on the immune system of the offspring.
T cells play a significant role in implantation, with the shift from Th1 to Th2 helper T cell response at the fetal–maternal interface contributing significantly to a successful pregnancy [104]. It is suggested that a Th2 shift inhibits Th1 cytokine responses, allowing the survival of the fetal allograft [105]. It has also been found that FAAH expression is regulated by the Th1 and Th2 cytokines, with IL-4 and IL-10 enhancing its activity and IL-2 and INF-γ reducing its expression [74]. A recent study has shown that expression of CB1 and CB2 receptors in B lymphocytes is differentially regulated during pregnancy [106]. Moreover, B cells from pregnant mice were shown to produce elevated levels of anti-inflammatory cytokine IL-10 following activation of CB1 receptors by select agonists [106]. Maternal exposure to synthetic cannabinoid HU-210 in rats was found to result in detectable changes in the development of the immune system, and long-lasting alterations to the functional status of the hypothalamus–pituitary–adrenal axis. Particularly, prenatal exposure to HU-210 caused a reduction in the T-helper subpopulation in the spleen and a dose-related decrease in the ratio of T helper/cytotoxic T cells in the peripheral blood of adult male offspring [107].
Murine fetal thymocytes express high levels of the CB1 and CB2 receptors [108]. Acute exposure to Δ9THC on gestation day 16 was shown to significantly impact fetal immune components as demonstrated by significant thymic atrophy and marked alterations in T cell subpopulations in fetuses on gestational days 16–18 as well as in pups on post-gestational day 1 [108]. Thymic atrophy was characterized by significant dose-dependent (20–50 mg/kg of Δ9THC) decrease in thymic cellularity which correlated with caspase-dependent apoptosis of thymocytes. Δ9THC exposure significantly decreased the number of single-positive CD8, double-positive CD4CD8 and double-negative T cell subsets of fetal thymocytes. Δ9THC (5–20 µM) also induced apoptosis in ex vivo fetal thymic organ cultures in a dose-dependent manner. These effects were mainly mediated by activation of CB1 and CB2 receptors as in vivo receptor blocking experiments using intraperitoneal injections of CB1 antagonist SR141716A (20 mg/kg) or CB2 antagonist AM630 (40 mg/kg) 1 h prior to Δ9THC (50 mg/kg) administration attenuated these immunological changes. Importantly, exposure to Δ9THC in pregnant mice had a significant and persistent effect on the postnatal immune response. For example, subchronic perinatal exposure to Δ9THC with the dosing regimen of 25 mg/kg on gestation day 16 and 10 mg/kg every day thereafter until the pups were born for a total of four injections resulted in significant decrease in thymic and splenic cellularity in 1-week-old offspring, thus negatively affecting the immune system of the progeny. Moreover, decreased proliferative and antibody responses to HIV gp120 antigens by peripheral T cells from the offspring demonstrated significant immune dysregulation. Thus, exposure to 20–50 mg/kg Δ9THC in pregnant mice seems to trigger profound T cell dysfunction in the developing fetus and the immune system of the offspring, thereby suggesting that cannabinoid exposure during pregnancy may cause significant and long-lasting effects on immune function [108].
Human epidemiological observations linking cannabis use, HIV immunity and development of AIDS have been contradictory. In a retrospective study that evaluated the link between cannabis use and sexually transmitted diseases in pregnant women entering prenatal care in which 86 women using only cannabis as an illicit substance were compared to 441 drug-free women with regards to the prevalence of gonorrhea, chlamydia, syphilis, human immunodeficiency virus (HIV), hepatitis B surface antigen, human papillomavirus, and herpes virus. No significant differences were found in the prevalence of these sexually transmitted infectious diseases between pregnant women who used cannabis and the drug-free pregnant women [109]. However, an association between cannabis use and HIV progression and the development of symptomatic AIDS was reported in homosexual men [110]. HIV positive men who progressed to AIDS and to have used cannabis for 3 months or more were more likely to have a lower percentage of CD4 T cells and a higher percentage of CD8 T cells [110]. However, several other studies reported no statistically significant links between cannabis or synthetic cannabinoids and HIV infection or associated immune parameters [111, 112]. For example, a randomized and placebo-controlled intervention trial involving 67 patients with HIV-1 infection, cannabis smoking and oral Dronabinol did not appear to adversely affect HIV RNA levels, CD4+ or CD8+ cell counts, or protease inhibitor levels [113]. In vitro studies on the effects of cannabinoids on HIV have also been contradictory. One study noted that several cannabinoid receptor agonists, including Δ9THC, may enhance HIV infection of a human T cell line [114], whereas others have reported that the synthetic cannabinoid receptor agonist WIN55,212-2 inhibited HIV expression in CD4 T lymphocytes and microglial cell cultures [115, 116]. However, in a hybrid mouse model in which human peripheral blood leukocytes were implanted into severe combined immunodeficient mice (huPBL-SCID), exposure to Δ9THC could suppress immune function, increase HIV co-receptor expression, and act as a cofactor to significantly enhance HIV replication [117]. HIV+ patients who were also cannabis users had lower circulating CD16 monocytes and IFN-γ-inducible protein 10 (IP-10) levels when compared to those not using cannabis [118]. Daily cannabis use was strongly associated with moderate to severe fibrosis in hepatitis C virus-infected individuals [119] and with liver fibrosis progression in patients with chronic hepatitis C [120]. The CB1 receptors were found to promote the progression of fibrosis as CB1 antagonism was able to attenuate liver fibrogenesis primarily by decreasing hepatic TGF-β [121]. CB1 and CB2 receptors have also been shown to play opposite roles in the pathogenesis of alcoholic liver disease via regulation of reinforcing properties of alcohol in the brain as well as hepatic cell injury and inflammation by endocannabinoids [122].
Immunomodulatory activity of both plant-derived and endocannabinoids have been also studied in animal models of inflammation such as allergic contact dermatitis, autoimmune hepatitis and graft-versus-host disease [14, 123–130]. Cannabinoids have been shown to typically act by suppressing proinflammatory cytokines, decreasing effector CD4/CD8 T cell population by inducing apoptosis and inhibiting their proliferation [124, 128]. Moreover, they can also upregulate certain chemokines or growth factors (G-CSF) and induce regulatory T cells or immunosuppressive myeloid cells [124, 127]. While most cannabinoids exert these effects via activation of cannabinoid receptors (CB1/CB2) [123, 124, 128, 130], CBD has been shown to function through vanilloid (Trpv1) receptors to ameliorate inflammation [127]. The robust anti-inflammatory activity of cannabinoids can have significant impacts during pregnancy. For example, use of non-steroidal anti-inflammatory drugs (NSAIDS) during pregnancy has been linked to miscarriages and other adverse outcome [131, 132]. According to a nested case-controlled study of 47,050 women, the risk of miscarriage was 2.4 times greater for those who took NSAIDS in early pregnancy [133]. The synthesis of prostaglandin is important in later stages of pregnancy and for fetal maturation [134]. Therefore, exposure to NSAIDS during the third trimester can affect fetal development and cause fetal ductal constriction [135]. Clinical evidence supports the association of changes in fetal ductus arteriosus flow and maternal consumption of foods rich in natural anti-inflammatory substances such as polyphenols [136–138]. Maternal dietary intervention during the third trimester of pregnancy by restricting potent anti-inflammatory foods for a period 2 weeks or more had a beneficial outcome with improved fetal ductal flow dynamics and reduced dimensions of the right ventricle [137]. Pro and anti-inflammatory conditions are tightly controlled in utero during pregnancy. Such fine-tuned regulation of inflammatory milieu is critical for optimal maintenance of pregnancy, fetal health, development and normal labor. While intrinsic changes in endocannabinoid levels appear to be an important component of this regulatory process, exposure to exogenous immunomodulatory cannabinoids could significantly alter this balance.
Increased frequency of mutant lymphocytes were observed in cannabis-smoking mothers and their newborns, suggesting a link between maternal cannabis smoking and somatic mutations, with a potentially elevated risk of developing malignancies [139]. Feinshtein et al., examined the influence of short-term exposure of human placental epithelial cell lines to CBD, and found that CBD inhibited placental breast cancer resistance protein function. Further, CBD significantly enhanced glyburide transport across human placenta ex vivo, suggesting that cannabinoids could enhance placental barrier permeability to other xenobiotics and endanger the developing fetus [140]. The association between maternal cannabis use and incidence of certain childhood malignancies such as rhabdomyosarcoma, astrocytoma and leukemia have been studied [141–144]. Trans-generational assessment of the effect of maternal cannabis use in 204 case-controlled women during and 1 year preceding pregnancy showed as much as an 11-fold increased risk of childhood acute non-lymphoblastic leukemia in offspring [141]. In cases of childhood acute myeloid leukemia, the risk of association was not observed with maternal cannabis use 3 months prior to or during pregnancy [89]. An evaluation of the self-reported use of recreational drugs in the mothers of 538 children with neuroblastoma showed that cannabis use during the first trimester of pregnancy was associated with significantly increased risk of neuroblastoma in the offspring, whereas its use during late pregnancy did not increase the risk. The association of gestational cannabis exposure with cancer incidence was particularly strong in children diagnosed with neuroblastoma before the age of 1 year [145]. However, these epidemiological studies have major limitations in that the data were mainly obtained by hospital surveys and information on the amount of exposure was not available or dose–response evaluations were not performed, and therefore, did not establish a causative link.
Potential impact of metabolites of exogenous cannabinoids
The detection of metabolites of Δ9THC and CBD in human hair and body fluids, and their precise quantitation methods have been well-developed particularly in the context of clinical toxicology and forensics of cannabis abuse [146–148]. However, unlike in the case of parent cannabinoids, there is limited literature on the biological activities of their metabolites.
11-Nor-9-carboxy-Δ9-tetrahydrocannabinol also known as THC-11-oic acid (11-COOH-THC) is the most abundant metabolite of Δ9THC [149–151]. As such 11-COOH-THC in body fluids is the clinical and forensic marker for cannabis exposure. Δ9THC is primarily metabolized by liver cytochrome P-450 (CYP450) isoenzymes into Phase I metabolites, which are oxidative and/or hydroxylated derivatives [152]. The initial oxidative metabolite is 11-hydroxy-Δ9THC (11-OH-THC), which is psychoactive. Further oxidative metabolism gives rise to 11-COOH-THC, which is inactive at CB1 and hence non-psychoactive [153]. This and other oxidized metabolites can also get converted to glucuronide esters as Phase II metabolites before excretion. 11-COOH-THC could be detected in the newborn meconium to determine the maternal exposure to cannabis [154], which suggested its placental transfer. Although 11-COOH-THC is psychotropically inactive, it exhibits analgesic and anti-inflammatory properties and hence considered as a biologically active metabolite. Similar to Δ9THC and CBD, 11-COOH-THC suppressed melatonin biosynthesis in rat pineal gland preparations ex vivo [155]. Orally administered 11-COOH-THC showed higher activity than Δ9THC in preventing platelet-mediated edema [156]. Moreover, 11-THC-COOH showed topical anti-inflammatory effects in vivo in experimental ear edema model in mice [157]. Certain synthetic cannabinoids such as ajulemic acid (AjA), endocannabinoids, and 11-THC-COOH have been shown to also influence eicosanoid biosynthesis. Endocannabinoids 2-AG and AEA can serve as a source of AA as well as metabolized by most eicosanoid biosynthetic enzymes, yielding additional lipids that regulate inflammatory cell functions [158]. AjA can increase the steady-state levels of COX2 mRNA and AA release, and can selectively and markedly upregulate 15d-PGJ2, an eicosanoid which facilitates resolution of inflammation [159]. While AjA could induce two- to sevenfold increase in the production of anti-inflammatory eicosanoid lipoxin A4 [160], 11-COOH-THC was found to inhibit cyclooxygenase and 5-lipoxygenase activities involved in prostaglandin biosynthesis, and hence decrease the production of proinflammatory prostaglandin eicosanoids [161].
Unlike Δ9THC, the metabolism of CBD is extremely complex, with more than 100 different metabolites identified [162]. The major metabolites of CBD are water soluble, hydroxylated 7-COOH derivatives [163]. Similar to natural enantiomer (−) CBD, the 7-COOH metabolites had very low affinities for CB1 and CB2 receptors. Whereas those derived from synthetic (+) CBD enantiomer exhibited high (Ki = 13.2 nM) and modest (Ki = 156 nM) affinities, respectively, for CB1 and CB2. Moreover, while both CBD enantiomers were good agonists of vanilloid (Trpv1) receptors, the 7-COOH metabolites showed no Trpv1 binding [164]. The 7-COOH CBD metabolites may also have anti-nociceptive and anti-inflammatory activities as they were found to inhibit the generation of nitric oxide and reactive oxygen species as well as production of TNF-α in vitro in a dose-dependent manner [164].
A number of human metabolites of synthetic cannabinoids have been reported [165, 166]. However, studies on their biological effects are limited. Mass spectrometric analysis of human urine specimens of individuals exposed to JWH-018 has identified monohydroxylated and carboxylated derivatives as the major metabolites, with hydroxylated primary metabolites exhibiting potent CB1 receptor agonistic activity [165, 167]. The metabolites of synthetic cannabinoids can also produce stronger activation of CB1 and CB2 receptors than Δ9THC, and may possess distinct pharmacology and higher toxicity [168–170].
While studies investigating the direct impact of metabolites of exogenous cannabinoids are lacking, their known biological activities as discussed above have the potential to interfere during pregnancy. It is likely that the immunosuppressive activity of bioactive cannabinoid metabolites may interfere with normal inflammatory changes and eicosanoid-prostaglandin homeostasis during pregnancy. Whether or not these metabolites affect fetal development or have specific neuronal and immune-related effects impacting the offspring needs further investigation.
Emerging role of epigenetic mechanisms
Epigenetics refers to stable, long-term alterations in the cell or individual that involves mechanisms of gene regulation by post-transcriptional and post-translational modifications, but not direct changes to the DNA sequence [171, 172]. The epigenetic regulatory machinery includes DNA methylation, histone modifications and non-coding RNAs. Recent studies have explored the impact of phytocannabinoids, synthetic cannabinoids and endocannabinoids on the epigenetic components [173–177].
Using a combined computational and experimental approach, it was shown that myocardial CB1 receptors were regulated by microRNA(miR)-494 and that CB2 receptors were targeted by miR-665, with miR-494 enhanced and miR-665 significantly repressed in chronic heart failure [178]. In Simian immunodeficiency virus-infected macaques, miRNA expression was profiled in intestines at 14, 30, and 60 days post-infection with or without chronic Δ9THC administration [179]. Chronic Δ9THC exposure was found to significantly increase the total number of differentially expressed miRNAs, selectively enhancing the expression of miR-10a, miR-24, miR-99b, miR-145, miR-149 and miR-187, that were found to target pro-inflammatory pathways, suggesting that the selective upregulation of anti-inflammatory miRNAs may contribute to Δ9THC-induced attenuation of gastrointestinal inflammation and maintenance of intestinal homeostasis [179].
The immunomodulatory effect of Δ9THC in experimental superantigen-elicited immune response in mice was shown to be mediated by epigenetic regulation [176]. In this study, changes in histone modifications in activated lymphocytes from mice following staphylococcal enterotoxin B superantigen challenge with or without Δ9THC administration were studied using ChIP-Seq approach. Global histone methylation and acetylation were found to be altered by Δ9THC, which caused an increase in active histone modification marks (H3K4me3) in Th2-associated genes and of suppressive modification signals (H3K27me3) in Th1-associated genes, suggesting for the first time that Δ9THC might modulate immune response through epigenetic histone modifications. In humans, the regulation of increased proenkephalin (Penk) expression, which is an opioid neuropeptide gene, was found to be mediated via decreased histone H3K9 methylation in the brain nucleus accumbens of adults following adolescent Δ9THC exposure, thereby disrupting the normal developmental pattern of this epigenetic mark. It was suggested that epigenetic dysregulation of Penk underlies the long-term effects of Δ9THC particularly in the neurobiological mechanisms of vulnerability to abuse of other drugs associated with cannabis abuse [180]. Rotter et al., have investigated the CB1/CB2 receptor promoter methylation status in peripheral blood cells of individuals with Δ9THC dependence and non-smoking control subjects. A significant negative correlation between mean promoter methylation frequency and CB1 expression was noted with a higher CB1 expression associated with cannabis consumption. Thus, altered CB1 expression associated with Δ9THC dependence was found to be mediated by changes to promoter methylation status [181].
Our seminal study demonstrated that cannabinoid receptor activation by Δ9THC in mice leads to a rapid and massive expansion of CD11b+Gr-1+ myeloid-derived suppressor cells (MDSC) expressing functional arginase and exhibiting potent immunosuppressive properties both ex vivo and upon adoptive transfer in vivo [182]. Further, the induction of MDSCs by Δ9THC in vivo was associated with robust upregulation of chemokines, particularly G-csf and Cxcl1. Thus, the induction of certain chemokines and MDSCs was identified as a major mechanism of immunomodulation by Δ9THC. MDSCs are the major immunosuppressive innate cell population induced in cancer, where they play a critical role in cancer immune escape [183, 184]. Epigenetic changes involving microRNA in MDSCs that are induced in vivo following exposure to Δ9THC have been studied in mice [175]. Δ9THC-induced MDSCs were found to exhibit distinct global microRNA expression profile compared to other myeloid cells and control bone marrow myeloid progenitor cells. The targets of differentially expressed miRNA were significantly associated with hematopoiesis and myeloid cell function Gene Ontology clusters as well as myeloid differentiation biological pathways. In fact, several of the altered miRNA were found to directly target crucial transcription factors involved in myeloid differentiation and function. Importantly, miRNA-690, highly overexpressed in Δ9THC-MDSCs, was found to target and regulate CCAAT/enhancer-binding protein α (C/EBPα), a master regulator of myeloid differentiation. The functional nature of this regulatory circuit was further confirmed by ex vivo miR-690 knockdown in primary MDSCs [175]. Moreover, endocannabinoid 2-AG has been recently shown to increase the presence and suppressive potency of MDSCs in brain [185], and AEA was shown to suppress Th17 cell-mediated experimental delayed type hypersensitivity response in vivo in mice by inducing IL-10 which in turn triggered a set of miRNA specifically targeting pro-inflammatory pathways [186].
Concluding remarks and perspective
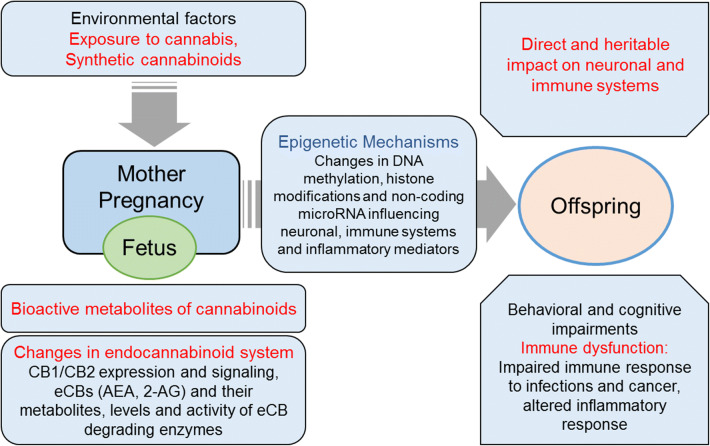
Currently accepted theories of the fetal origins of adult diseases involve in utero exposure and response to environmental factors that ultimately lead to persistent effects with increased susceptibility to certain diseases later in life. Although precise mechanisms are not completely known, accumulating evidence in recent years suggests the involvement of epigenetic regulatory pathways [187, 188]. Studies on the effects of cannabinoids on the epigenome thus far have been mostly performed in adults. As epigenetic changes are stable and have sustained effects, these early results suggest that cannabis abuse could have a trans-generational impact, and that such events early in life in utero might have a significant impact on fetal health and progeny, including the critical immune components (Fig. 2). Importantly, immune system and inflammation also plays a critical role in the etiology of number of neurological and psychiatric illnesses. Women during pregnancy could potentially get exposed to high potency cannabis and synthetic cannabinoids. Robust controlled human studies involving drug abuse patients on the specific effects of cannabis and synthetic cannabinoids on immune function in neonates and offspring are lacking. Future studies with a balanced approach are needed to examine the dose and duration-dependent effects cannabis components and synthetic cannabinoids on immune function and other health aspects to further clearly understand their harmful impact and pregnancy risks, as well as any potential beneficial effects.
Fig. 2.
Potential health impacts of cannabis and synthetic cannabinoid abuse during pregnancy. Cannabinoids are known to cross the maternofetal placental barrier. Maternal exposure to cannabinoids may exert direct effects as well as via their bioactive metabolites or by dysregulation of endocannabinoid (eCB) signaling. This can have a long-lasting impact on the developing fetus and offspring with significant neuronal impairment and immune dysfunction involving epigenetic mechanisms
Acknowledgements
Catherine Dong and Amy Harrington received the Magellan Fellowships from the University of South Carolina. The authors’ research on cannabinoids was supported by the US National Institutes of Health (Grants DA034892 to VLH and DA020531 to KYV).
References
- 1.Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Curr Opin Psychiatry. 2007;20:393–397. doi: 10.1097/YCO.0b013e32812144cc. [DOI] [PubMed] [Google Scholar]
- 2.NIDA (2018) Drug facts: Marijuana. National Institute on Drug Abuse, Bethesda. https://www.drugabuse.gov/publications/drugfacts/marijuana. Accessed 31 Aug 2018
- 3.Freund SA, Banning AS. Synthetic cannabinoids: a review of the clinical implications of a new drug of choice. JAAPA. 2017;30:1–4. doi: 10.1097/01.JAA.0000525914.28344.e2. [DOI] [PubMed] [Google Scholar]
- 4.Schrot RJ, Hubbard JR. Cannabinoids: medical implications. Ann Med. 2016;48:128–141. doi: 10.3109/07853890.2016.1145794. [DOI] [PubMed] [Google Scholar]
- 5.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313:2474–2483. doi: 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- 6.Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- 7.Svrakic DM, Lustman PJ, Mallya A, et al. Legalization, decriminalization and medicinal use of cannabis: a scientific and public health perspective. Mol Med. 2012;109:90–98. [PMC free article] [PubMed] [Google Scholar]
- 8.Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1614. doi: 10.1046/j.1360-0443.1996.911115852.x. [DOI] [PubMed] [Google Scholar]
- 9.ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Zuardi AW, Crippa JAS, Hallak JEC, et al. A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;18:5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
- 11.Di Marzo V, Petrocellis LD. Plant, synthetic, and endogenous cannabinoids in medicine. Annu Rev Med. 2006;57:553–574. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- 12.Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373:1048–1058. doi: 10.1056/NEJMra1407304. [DOI] [PubMed] [Google Scholar]
- 13.Pertwee RG. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci. 2012;367:3353–3363. doi: 10.1098/rstb.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagarkatti P, Pandey R, Rieder SA, et al. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO (2016) Management of substance abuse: Cannabis. World Health Organisation. http://www.who.int/substance_abuse/facts/cannabis/en.2016. Accessed 31 Aug 2018
- 16.Johnston L, O’Malley P, Bachman J, Schulenberg J. Monitoring the Future national results on adolescent drug use: overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- 17.Azofeifa A, Mattson ME, Schauer G, et al. National estimates of marijuana use and related indicators—National Survey on Drug Use and Health, United States, 2002–2014. MMWR Surveill Summ. 2016;65:1–28. doi: 10.15585/mmwr.ss6511a1. [DOI] [PubMed] [Google Scholar]
- 18.SAMHSA (2017) Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health: Methodological resource book (Section 13, Statistical inference report). Substance Abuse and Mental Health Services Administration, Rockville. https://www.samhsa.gov/data. Accessed 31 Aug 2018
- 19.NIDA (2018) Drug facts: synthetic cannabinoids (K2/Spice). National Institute on Drug Abuse. https://www.drugabuse.gov/publications/drugfacts/synthetic-cannabinoids-k2spice. Accessed 30 Mar 2018
- 20.Wu L-T, Zhu H, Swartz MS. Trends in cannabis use disorders among racial/ethnic population groups in the United States. Drug Alcohol Depend. 2016;165:181–190. doi: 10.1016/j.drugalcdep.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T. 2017;42:180–188. [PMC free article] [PubMed] [Google Scholar]
- 22.Cascini F, Aiello C, Di Tanna G. Increasing delta-9-tetrahydrocannabinol (Δ-9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr Drug Abuse Rev. 2012;5:32–40. doi: 10.2174/1874473711205010032. [DOI] [PubMed] [Google Scholar]
- 23.Loeffler G, Delaney E, Hann M. International trends in spice use: prevalence, motivation for use, relationship to other substances, and perception of use and safety for synthetic cannabinoids. Brain Res Bull. 2016;126:8–28. doi: 10.1016/j.brainresbull.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Palamar JJ, Acosta P. Synthetic cannabinoid use in a nationally representative sample of US high school seniors. Drug Alcohol Depend. 2015;149:194–202. doi: 10.1016/j.drugalcdep.2015.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fattore L, Fratta W. Beyond THC: the new generation of cannabinoid designer drugs. Front Behav Neurosci. 2011;5:60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castaneto MS, Gorelick DA, Desrosiers NA, et al. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper ZD. Adverse effects of synthetic cannabinoids: management of acute toxicity and withdrawal. Curr Psychiatry Rep. 2016;18:52. doi: 10.1007/s11920-016-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gudsoorkar VS, Perez JA. A new differential diagnosis: synthetic cannabinoids-associated acute renal failure. Methodist Debakey Cardiovasc J. 2015;11:189–191. doi: 10.14797/mdcj-11-3-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford BM, Tai S, Fantegrossi WE, Prather PL. Synthetic pot: not your grandfather’s marijuana. Trends Pharmacol Sci. 2017;38:257–276. doi: 10.1016/j.tips.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felder CC, Joyce KE, Briley EM, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- 31.Grotenhermen F. Pharmacology of cannabinoids. Neuro Endocrinol Lett. 2004;25:14–23. [PubMed] [Google Scholar]
- 32.Johnston M, O’Malley P, Bachman J (2000) Monitoring the future national survey results on drug use, 1975–1999. Volume II: College Students and Adults Ages 19–40. National Institute of Drug Abuse, Rockville. http://www.monitoringthefuture.org/pubs.html. Accessed 5 Oct 2018
- 33.Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996–1998. Obstet Gynecol. 2003;101:374–379. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- 34.Lipari RN, Hedden SL, Hughes A (2013) Substance use and mental health estimates from the 2013 National Survey on Drug Use and Health: Overview of Findings. In: The CBHSQ Report. Substance Abuse and Mental Health Services Administration (US), Rockville [PubMed]
- 35.Gunn JKL, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6:e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SAMHSA . Results from the 2013 National Survey on Drug Use and Health: summary of national findings. Rockville: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 37.Martin CE, Longinaker N, Mark K, et al. Recent trends in treatment admissions for marijuana use during pregnancy. J Addict Med. 2015;9:99–104. doi: 10.1097/ADM.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 38.Braillon A, Bewley S. Committee Opinion No. 722: marijuana use during pregnancy and lactation. Obstet Gynecol. 2018;131:164. doi: 10.1097/AOG.0000000000002429. [DOI] [PubMed] [Google Scholar]
- 39.Brancato A, Cannizzaro C. Mothering under the influence: how perinatal drugs of abuse alter the mother–infant interaction. Rev Neurosci. 2018;29:283–294. doi: 10.1515/revneuro-2017-0052. [DOI] [PubMed] [Google Scholar]
- 40.Alpár A, Di Marzo V, Harkany T. At the tip of an iceberg: prenatal marijuana and its possible relation to neuropsychiatric outcome in the offspring. Biol Psychiatry. 2016;79:e33–e45. doi: 10.1016/j.biopsych.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Campolongo P, Trezza V, Palmery M, et al. Developmental exposure to cannabinoids causes subtle and enduring neurofunctional alterations. Int Rev Neurobiol. 2009;85:117–133. doi: 10.1016/S0074-7742(09)85009-5. [DOI] [PubMed] [Google Scholar]
- 42.Hayatbakhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215–219. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- 43.Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci. 2009;259:395–412. doi: 10.1007/s00406-009-0027-z. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Dow-Edwards D, Anderson V, et al. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. 2004;56:909–915. doi: 10.1016/j.biopsych.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Brancato A, Lavanco G, Cavallaro A, et al. The use of the emotional-object recognition as an assay to assess learning and memory associated to an aversive stimulus in rodents. J Neurosci Methods. 2016;274:106–115. doi: 10.1016/j.jneumeth.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Brancato A, Cavallaro A, Lavanco G, et al. Reward-related limbic memory and stimulation of the cannabinoid system: an upgrade in value attribution? J Psychopharmacol (Oxford) 2018;32:204–214. doi: 10.1177/0269881117725683. [DOI] [PubMed] [Google Scholar]
- 47.Fergusson DM, Horwood LJ, Northstone K, ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood Maternal use of cannabis and pregnancy outcome. BJOG. 2002;109:21–27. doi: 10.1111/j.1471-0528.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 48.Metz TD, Allshouse AA, Hogue CJ, et al. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am J Obstet Gynecol. 2017;217:478.e1–478.e8. doi: 10.1016/j.ajog.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devane WA. New dawn of cannabinoid pharmacology. Trends Pharmacol Sci. 1994;15:40–41. doi: 10.1016/0165-6147(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 51.Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/S0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 52.Luchicchi A, Pistis M. Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol Neurobiol. 2012;46:374–392. doi: 10.1007/s12035-012-8299-0. [DOI] [PubMed] [Google Scholar]
- 53.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cravatt BF, Giang DK, Mayfield SP, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 55.Bari M, Battista N, Pirazzi V, Maccarrone M. The manifold actions of endocannabinoids on female and male reproductive events. Front Biosci (Landmark Ed) 2011;16:498–516. doi: 10.2741/3701. [DOI] [PubMed] [Google Scholar]
- 56.Taylor AH, Amoako AA, Bambang K, et al. Endocannabinoids and pregnancy. Clin Chim Acta. 2010;411:921–930. doi: 10.1016/j.cca.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Battista N, Pasquariello N, Di Tommaso M, Maccarrone M. Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J Neuroendocrinol. 2008;20(Suppl 1):82–89. doi: 10.1111/j.1365-2826.2008.01684.x. [DOI] [PubMed] [Google Scholar]
- 58.Taylor AH, Ang C, Bell SC, Konje JC. The role of the endocannabinoid system in gametogenesis, implantation and early pregnancy. Hum Reprod Update. 2007;13:501–513. doi: 10.1093/humupd/dmm018. [DOI] [PubMed] [Google Scholar]
- 59.Fride E. Multiple roles for the endocannabinoid system during the earliest stages of life: pre- and postnatal development. J Neuroendocrinol. 2008;20(Suppl 1):75–81. doi: 10.1111/j.1365-2826.2008.01670.x. [DOI] [PubMed] [Google Scholar]
- 60.Maccarrone M, Valensise H, Bari M, et al. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet. 2000;355:1326–1329. doi: 10.1016/S0140-6736(00)02115-2. [DOI] [PubMed] [Google Scholar]
- 61.Maccarrone M, Bisogno T, Valensise H, et al. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Mol Hum Reprod. 2002;8:188–195. doi: 10.1093/molehr/8.2.188. [DOI] [PubMed] [Google Scholar]
- 62.Habayeb OMH, Taylor AH, Bell SC, et al. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149:5052–5060. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- 63.Maccarrone M, Finazzi-Agrò A. Anandamide hydrolase: a guardian angel of human reproduction? Trends Pharmacol Sci. 2004;25:353–357. doi: 10.1016/j.tips.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Schmid PC, Paria BC, Krebsbach RJ, et al. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 1997;94:4188–4192. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Almada M, Amaral C, Diniz-da-Costa M, et al. The endocannabinoid anandamide impairs in vitro decidualization of human cells. Reproduction. 2016;152:351–361. doi: 10.1530/REP-16-0364. [DOI] [PubMed] [Google Scholar]
- 66.Fonseca BM, Correia-da-Silva G, Teixeira NA. Anandamide restricts uterine stromal differentiation and is critical for complete decidualization. Mol Cell Endocrinol. 2015;411:167–176. doi: 10.1016/j.mce.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 67.Schuel H, Burkman LJ, Lippes J, et al. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol Reprod Dev. 2002;63:376–387. doi: 10.1002/mrd.90021. [DOI] [PubMed] [Google Scholar]
- 68.McIntosh AL, Martin GG, Huang H, et al. Δ9-Tetrahydrocannabinol induces endocannabinoid accumulation in mouse hepatocytes: antagonism by Fabp1 gene ablation. J Lipid Res. 2018;59:646–657. doi: 10.1194/jlr.M082644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leishman E, Murphy M, Mackie K, Bradshaw HB. Δ9-Tetrahydrocannabinol changes the brain lipidome and transcriptome differentially in the adolescent and the adult. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:479–492. doi: 10.1016/j.bbalip.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Hum Reprod Update. 2011;17:347–361. doi: 10.1093/humupd/dmq058. [DOI] [PubMed] [Google Scholar]
- 71.Maia J, Almada M, Silva A, et al. The endocannabinoid system expression in the female reproductive tract is modulated by estrogen. J Steroid Biochem Mol Biol. 2017;174:40–47. doi: 10.1016/j.jsbmb.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 72.Maccarrone M, Di Rienzo M, Finazzi-Agrò A, Rossi A. Leptin activates the anandamide hydrolase promoter in human T lymphocytes through STAT3. J Biol Chem. 2003;278:13318–13324. doi: 10.1074/jbc.M211248200. [DOI] [PubMed] [Google Scholar]
- 73.Maccarrone M, Bari M, Di Rienzo M, et al. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem. 2003;278:32726–32732. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]
- 74.Maccarrone M, Valensise H, Bari M, et al. Progesterone up-regulates anandamide hydrolase in human lymphocytes: role of cytokines and implications for fertility. J Immunol. 2001;166:7183–7189. doi: 10.4049/jimmunol.166.12.7183. [DOI] [PubMed] [Google Scholar]
- 75.Bambang KN, Lambert DG, Lam PMW, et al. Immunity and early pregnancy events: are endocannabinoids the missing link? J Reprod Immunol. 2012;96:8–18. doi: 10.1016/j.jri.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Psychoyos D, Vinod KY. Marijuana, Spice “herbal high”, and early neural development: implications for rescheduling and legalization. Drug Test Anal. 2013;5:27–45. doi: 10.1002/dta.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Psychoyos D, Vinod KY, Cao J, et al. Cannabinoid receptor 1 signaling in embryo neurodevelopment. Birth Defects Res B Dev Reprod Toxicol. 2012;95:137–150. doi: 10.1002/bdrb.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oh H-A, Kwon S, Choi S, et al. Uncovering a role for endocannabinoid signaling in autophagy in preimplantation mouse embryos. Mol Hum Reprod. 2013;19:93–101. doi: 10.1093/molehr/gas049. [DOI] [PubMed] [Google Scholar]
- 79.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update. 2003;9:347–357. doi: 10.1093/humupd/dmg026. [DOI] [PubMed] [Google Scholar]
- 80.Tafuri A, Alferink J, Möller P, et al. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- 81.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 82.Luppi P, Haluszczak C, Trucco M, Deloia JA. Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol. 2002;47:72–81. doi: 10.1034/j.1600-0897.2002.1o041.x. [DOI] [PubMed] [Google Scholar]
- 83.Luppi P, Haluszczak C, Betters D, et al. Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol. 2002;72:874–884. [PubMed] [Google Scholar]
- 84.Ueda Y, Hagihara M, Okamoto A, et al. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum Immunol. 2003;64:1144–1151. doi: 10.1016/j.humimm.2003.08.342. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y, Ling B, Zhou Y, et al. Interferon-gamma expression in natural killer cells and natural killer T cells is suppressed in early pregnancy. Cell Mol Immunol. 2007;4:389–394. [PubMed] [Google Scholar]
- 86.Harbison RD, Mantilla-Plata B. Prenatal toxicity, maternal distribution and placental transfer of tetrahydrocannabinol. J Pharmacol Exp Ther. 1972;180:446–453. [PubMed] [Google Scholar]
- 87.Vardaris RM, Weisz DJ, Fazel A, Rawitch AB. Chronic administration of delta-9-tetrahydrocannabinol to pregnant rats: studies of pup behavior and placental transfer. Pharmacol Biochem Behav. 1976;4:249–254. doi: 10.1016/0091-3057(76)90236-7. [DOI] [PubMed] [Google Scholar]
- 88.Blackard C, Tennes K. Human placental transfer of cannabinoids. N Engl J Med. 1984;311:797. doi: 10.1056/NEJM198409203111213. [DOI] [PubMed] [Google Scholar]
- 89.Bailey JR, Cunny HC, Paule MG, Slikker W. Fetal disposition of delta 9-tetrahydrocannabinol (THC) during late pregnancy in the rhesus monkey. Toxicol Appl Pharmacol. 1987;90:315–321. doi: 10.1016/0041-008X(87)90338-3. [DOI] [PubMed] [Google Scholar]
- 90.El Marroun H, Tiemeier H, Steegers EAP, et al. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J Am Acad Child Adolesc Psychiatry. 2009;48:1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- 91.Hurd YL, Wang X, Anderson V, et al. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol Teratol. 2005;27:221–229. doi: 10.1016/j.ntt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Murphy LL, Gher J, Szary A. Effects of prenatal exposure to delta-9-tetrahydrocannabinol on reproductive, endocrine and immune parameters of male and female rat offspring. Endocrine. 1995;3:875–879. doi: 10.1007/BF02738892. [DOI] [PubMed] [Google Scholar]
- 93.Cabral GA, Rogers TJ, Lichtman AH. Turning over a new leaf: cannabinoid and endocannabinoid modulation of immune function. J Neuroimmune Pharmacol. 2015;10:193–203. doi: 10.1007/s11481-015-9615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roth MD, Baldwin GC, Tashkin DP. Effects of delta-9-tetrahydrocannabinol on human immune function and host defense. Chem Phys Lipids. 2002;121:229–239. doi: 10.1016/S0009-3084(02)00159-7. [DOI] [PubMed] [Google Scholar]
- 95.Kaminski NE. Immune regulation by cannabinoid compounds through the inhibition of the cyclic AMP signaling cascade and altered gene expression. Biochem Pharmacol. 1996;52:1133–1140. doi: 10.1016/0006-2952(96)00480-7. [DOI] [PubMed] [Google Scholar]
- 96.Klein TW, Newton C, Larsen K, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 97.Galiègue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 98.Howlett AC, Bidaut-Russell M, Devane WA, et al. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-S. [DOI] [PubMed] [Google Scholar]
- 99.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 100.Kenney SP, Kekuda R, Prasad PD, et al. Cannabinoid receptors and their role in the regulation of the serotonin transporter in human placenta. Am J Obstet Gynecol. 1999;181:491–497. doi: 10.1016/S0002-9378(99)70583-1. [DOI] [PubMed] [Google Scholar]
- 101.Dennedy MC, Friel AM, Houlihan DD, et al. Cannabinoids and the human uterus during pregnancy. Am J Obstet Gynecol. 2004;190:2–9. doi: 10.1016/j.ajog.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 102.Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci USA. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Talatini MR, Taylor AH, Elson JC, et al. Localisation and function of the endocannabinoid system in the human ovary. PLoS One. 2009;4:e4579. doi: 10.1371/journal.pone.0004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piccinni MP, Beloni L, Livi C, et al. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 105.Piccinni MP, Romagnani S. Regulation of fetal allograft survival by a hormone-controlled Th1- and Th2-type cytokines. Immunol Res. 1996;15:141–150. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- 106.Wolfson ML, Muzzio DO, Ehrhardt J, et al. Expression analysis of cannabinoid receptors 1 and 2 in B cells during pregnancy and their role on cytokine production. J Reprod Immunol. 2016;116:23–27. doi: 10.1016/j.jri.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 107.del Arco I, Muñoz R, Rodríguez De Fonseca F, et al. Maternal exposure to the synthetic cannabinoid HU-210: effects on the endocrine and immune systems of the adult male offspring. Neuroimmunomodulation. 2000;7:16–26. doi: 10.1159/000026416. [DOI] [PubMed] [Google Scholar]
- 108.Lombard C, Hegde VL, Nagarkatti M, Nagarkatti PS. Perinatal exposure to Delta9-tetrahydrocannabinol triggers profound defects in T cell differentiation and function in fetal and postnatal stages of life, including decreased responsiveness to HIV antigens. J Pharmacol Exp Ther. 2011;339:607–617. doi: 10.1124/jpet.111.181206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller JM, Goodridge C. Antenatal marijuana use is unrelated to sexually transmitted infections during pregnancy. Infect Dis Obstet Gynecol. 2000;8:155–157. doi: 10.1155/S106474490000020X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tindall B, Cooper DA, Donovan B, et al. The Sydney AIDS Project: development of acquired immunodeficiency syndrome in a group of HIV seropositive homosexual men. Aust N Z J Med. 1988;18:8–15. doi: 10.1111/j.1445-5994.1988.tb02232.x. [DOI] [PubMed] [Google Scholar]
- 111.Bredt BM, Higuera-Alhino D, Shade SB, et al. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol. 2002;42:82S–89S. doi: 10.1002/j.1552-4604.2002.tb06007.x. [DOI] [PubMed] [Google Scholar]
- 112.Di Franco MJ, Sheppard HW, Hunter DJ, et al. The lack of association of marijuana and other recreational drugs with progression to AIDS in the San Francisco Men’s Health Study. Ann Epidemiol. 1996;6:283–289. doi: 10.1016/S1047-2797(96)00022-1. [DOI] [PubMed] [Google Scholar]
- 113.Abrams DI, Hilton JF, Leiser RJ, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003;139:258–266. doi: 10.7326/0003-4819-139-4-200308190-00008. [DOI] [PubMed] [Google Scholar]
- 114.Noe SN, Nyland SB, Ugen K, et al. Cannabinoid receptor agonists enhance syncytia formation in MT-2 cells infected with cell free HIV-1MN. Adv Exp Med Biol. 1998;437:223–229. doi: 10.1007/978-1-4615-5347-2_25. [DOI] [PubMed] [Google Scholar]
- 115.Peterson PK, Gekker G, Hu S, et al. Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures. J Neuroimmunol. 2004;147:123–126. doi: 10.1016/j.jneuroim.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 116.Rock RB, Gekker G, Hu S, et al. WIN55,212-2-mediated inhibition of HIV-1 expression in microglial cells: involvement of cannabinoid receptors. J Neuroimmune Pharmacol. 2007;2:178–183. doi: 10.1007/s11481-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 117.Roth MD, Tashkin DP, Whittaker KM, et al. Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci. 2005;77:1711–1722. doi: 10.1016/j.lfs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 118.Rizzo MD, Crawford RB, Henriquez JE, et al. HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-γ-inducible protein 10 levels compared with nonusing HIV patients. AIDS. 2018;32:419–429. doi: 10.1097/QAD.0000000000001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ishida JH, Peters MG, Jin C, et al. Influence of cannabis use on severity of hepatitis C disease. Clin Gastroenterol Hepatol. 2008;6:69–75. doi: 10.1016/j.cgh.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42:63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- 121.Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 122.Lavanco G, Castelli V, Brancato A, et al. The endocannabinoid-alcohol crosstalk: recent advances on a bi-faceted target. Clin Exp Pharmacol Physiol. 2018 doi: 10.1111/1440-1681.12967. [DOI] [PubMed] [Google Scholar]
- 123.Karsak M, Gaffal E, Date R, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- 124.Hegde VL, Hegde S, Cravatt BF, et al. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pandey R, Hegde VL, Singh NP, et al. Use of cannabinoids as a novel therapeutic modality against autoimmune hepatitis. Vitam Horm. 2009;81:487–504. doi: 10.1016/S0083-6729(09)81019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagarkatti M, Rieder SA, Hegde VL, et al. Do cannabinoids have a therapeutic role in transplantation? Trends Pharmacol Sci. 2010;31:345–350. doi: 10.1016/j.tips.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hegde VL, Nagarkatti PS, Nagarkatti M. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PLoS One. 2011;6:e18281. doi: 10.1371/journal.pone.0018281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pandey R, Hegde VL, Nagarkatti M, Nagarkatti PS. Targeting cannabinoid receptors as a novel approach in the treatment of graft-versus-host disease: evidence from an experimental murine model. J Pharmacol Exp Ther. 2011;338:819–828. doi: 10.1124/jpet.111.182717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Katchan V, David P, Shoenfeld Y. Cannabinoids and autoimmune diseases: a systematic review. Autoimmun Rev. 2016;15:513–528. doi: 10.1016/j.autrev.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 130.Baker D, Jackson SJ, Pryce G. Cannabinoid control of neuroinflammation related to multiple sclerosis. Br J Pharmacol. 2007;152:649–654. doi: 10.1038/sj.bjp.0707458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Antonucci R, Zaffanello M, Puxeddu E, et al. Use of non-steroidal anti-inflammatory drugs in pregnancy: impact on the fetus and newborn. Curr Drug Metab. 2012;13:474–490. doi: 10.2174/138920012800166607. [DOI] [PubMed] [Google Scholar]
- 132.Li D-K, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327:368. doi: 10.1136/bmj.327.7411.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakhai-Pour HR, Broy P, Sheehy O, Bérard A. Use of nonaspirin nonsteroidal anti-inflammatory drugs during pregnancy and the risk of spontaneous abortion. CMAJ. 2011;183:1713–1720. doi: 10.1503/cmaj.110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Young IR, Thorburn GD. Prostaglandin E2, fetal maturation and ovine parturition. Aust N Z J Obstet Gynaecol. 1994;34:342–346. doi: 10.1111/j.1479-828X.1994.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 135.Koren G, Florescu A, Costei AM, et al. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Ann Pharmacother. 2006;40:824–829. doi: 10.1345/aph.1G428. [DOI] [PubMed] [Google Scholar]
- 136.Hahn M, Baierle M, Charão MF, et al. Polyphenol-rich food general and on pregnancy effects: a review. Drug Chem Toxicol. 2017;40:368–374. doi: 10.1080/01480545.2016.1212365. [DOI] [PubMed] [Google Scholar]
- 137.Zielinsky P, Piccoli AL, Vian I, et al. Maternal restriction of polyphenols and fetal ductal dynamics in normal pregnancy: an open clinical trial. Arq Bras Cardiol. 2013;101:217–225. doi: 10.5935/abc.20130166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vian I, Zielinsky P, Zílio AM, et al. Increase of prostaglandin E2 in the reversal of ductal constriction after polyphenol restriction. Ultrasound Obstet Gynecol. 2017 doi: 10.1002/uog.18974. [DOI] [PubMed] [Google Scholar]
- 139.Ammenheuser MM, Berenson AB, Babiak AE, et al. Frequencies of hprt mutant lymphocytes in marijuana-smoking mothers and their newborns. Mutat Res. 1998;403:55–64. doi: 10.1016/S0027-5107(98)00027-X. [DOI] [PubMed] [Google Scholar]
- 140.Feinshtein V, Erez O, Ben-Zvi Z, et al. Cannabidiol enhances xenobiotic permeability through the human placental barrier by direct inhibition of breast cancer resistance protein: an ex vivo study. Am J Obstet Gynecol. 2013;209:573.e1–573.e15. doi: 10.1016/j.ajog.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 141.Robison LL, Buckley JD, Daigle AE, et al. Maternal drug use and risk of childhood nonlymphoblastic leukemia among offspring. An epidemiologic investigation implicating marijuana (a report from the Childrens Cancer Study Group) Cancer. 1989;63:1904–1911. [PubMed] [Google Scholar]
- 142.Grufferman S, Schwartz AG, Ruymann FB, Maurer HM. Parents’ use of cocaine and marijuana and increased risk of rhabdomyosarcoma in their children. Cancer Causes Control. 1993;4:217–224. doi: 10.1007/BF00051316. [DOI] [PubMed] [Google Scholar]
- 143.Kuijten RR, Bunin GR, Nass CC, Meadows AT. Gestational and familial risk factors for childhood astrocytoma: results of a case–control study. Cancer Res. 1990;50:2608–2612. [PubMed] [Google Scholar]
- 144.Hall W, MacPhee D. Cannabis use and cancer. Addiction. 2002;97:243–247. doi: 10.1046/j.1360-0443.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- 145.Bluhm EC, Daniels J, Pollock BH, et al. Maternal use of recreational drugs and neuroblastoma in offspring: a report from the Children’s Oncology Group (United States) Cancer Causes Control. 2006;17:663–669. doi: 10.1007/s10552-005-0580-3. [DOI] [PubMed] [Google Scholar]
- 146.Moeller MR, Doerr G, Warth S. Simultaneous quantitation of delta-9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in serum by GC/MS using deuterated internal standards and its application to a smoking study and forensic cases. J Forensic Sci. 1992;37:969–983. doi: 10.1520/JFS13282J. [DOI] [PubMed] [Google Scholar]
- 147.Frazee CC, Kiscoan M, Garg U. Quantitation of total 11-nor-9-carboxy-delta 9-tetrahydrocannabinol in urine and blood using gas chromatography-mass spectrometry (GC–MS) Methods Mol Biol. 2010;603:137–144. doi: 10.1007/978-1-60761-459-3_13. [DOI] [PubMed] [Google Scholar]
- 148.Fu S, Lewis J. Novel automated extraction method for quantitative analysis of urinary 11-nor-delta(9)-tetrahydrocannabinol-9-carboxylic acid (THC-COOH) J Anal Toxicol. 2008;32:292–297. doi: 10.1093/jat/32.4.292. [DOI] [PubMed] [Google Scholar]
- 149.Wall ME, Perez-Reyes M. The metabolism of delta 9-tetrahydrocannabinol and related cannabinoids in man. J Clin Pharmacol. 1981;21:178S–189S. doi: 10.1002/j.1552-4604.1981.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 150.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schwilke EW, Schwope DM, Karschner EL, et al. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin Chem. 2009;55:2180–2189. doi: 10.1373/clinchem.2008.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46:86–95. doi: 10.3109/03602532.2013.849268. [DOI] [PubMed] [Google Scholar]
- 153.Sharma P, Murthy P, Bharath MMS. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry. 2012;7:149–156. [PMC free article] [PubMed] [Google Scholar]
- 154.de Mantovani C, Silva JPE, Forster G, et al. Simultaneous accelerated solvent extraction and hydrolysis of 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid glucuronide in meconium samples for gas chromatography-mass spectrometry analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1074–1075:1–7. doi: 10.1016/j.jchromb.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 155.Koch M, Dehghani F, Habazettl I, et al. Cannabinoids attenuate norepinephrine-induced melatonin biosynthesis in the rat pineal gland by reducing arylalkylamine N-acetyltransferase activity without involvement of cannabinoid receptors. J Neurochem. 2006;98:267–278. doi: 10.1111/j.1471-4159.2006.03873.x. [DOI] [PubMed] [Google Scholar]
- 156.Burstein SH, Audette CA, Doyle SA, et al. Antagonism to the actions of platelet activating factor by a nonpsychoactive cannabinoid. J Pharmacol Exp Ther. 1989;251:531–535. [PubMed] [Google Scholar]
- 157.Tius MA, Kannangara GSK, Kerr MA, Grace KJS. Halogenated cannabinoid synthesis. Tetrahedron. 1993;49:3291–3304. doi: 10.1016/S0040-4020(01)90158-9. [DOI] [Google Scholar]
- 158.Turcotte C, Chouinard F, Lefebvre JS, Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol. 2015;97:1049–1070. doi: 10.1189/jlb.3RU0115-021R. [DOI] [PubMed] [Google Scholar]
- 159.Stebulis JA, Johnson DR, Rossetti RG, et al. Ajulemic acid, a synthetic cannabinoid acid, induces an antiinflammatory profile of eicosanoids in human synovial cells. Life Sci. 2008;83:666–670. doi: 10.1016/j.lfs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 160.Zurier RB, Sun Y-P, George KL, et al. Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. FASEB J. 2009;23:1503–1509. doi: 10.1096/fj.08-118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takeda S, Jiang R, Aramaki H, et al. Δ9-tetrahydrocannabinol and its major metabolite Δ9-tetrahydrocannabinol-11-oic acid as 15-lipoxygenase inhibitors. J Pharm Sci. 2011;100:1206–1211. doi: 10.1002/jps.22354. [DOI] [PubMed] [Google Scholar]
- 162.Harvey D. Metabolism and pharmacokinetics of the cannabinoids. In: Watson RR, editor. Biochemistry and physiology of substance abuse. Boca Raton: CRC Press; 1991. pp. 279–365. [Google Scholar]
- 163.Ujváry I, Hanuš L. Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 2016;1:90–101. doi: 10.1089/can.2015.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mechoulam R, Tchilibon S et al (2010) United States Patent: 7759526—pharmaceutical compositions comprising cannabidiol derivatives. http://patft.uspto.gov. Accessed 5 Oct 2018
- 165.Chimalakonda KC, Seely KA, Bratton SM, et al. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40:2174–2184. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.ElSohly MA, Gul W, Elsohly KM, et al. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35:487–495. doi: 10.1093/anatox/35.7.487. [DOI] [PubMed] [Google Scholar]
- 167.Brents LK, Reichard EE, Zimmerman SM, et al. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014;97:45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tai S, Fantegrossi WE. Pharmacological and toxicological effects of synthetic cannabinoids and their metabolites. Curr Top Behav Neurosci. 2017;32:249–262. doi: 10.1007/7854_2016_60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rajasekaran M, Brents LK, Franks LN, et al. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol. 2013;269:100–108. doi: 10.1016/j.taap.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 172.Martin C, Zhang Y. Mechanisms of epigenetic inheritance. Curr Opin Cell Biol. 2007;19:266–272. doi: 10.1016/j.ceb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 173.Szutorisz H, Hurd YL. Epigenetic effects of cannabis exposure. Biol Psychiatry. 2016;79:586–594. doi: 10.1016/j.biopsych.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zumbrun EE, Sido JM, Nagarkatti PS, Nagarkatti M. Epigenetic regulation of immunological alterations following prenatal exposure to marijuana cannabinoids and its long term consequences in offspring. J Neuroimmune Pharmacol. 2015;10:245–254. doi: 10.1007/s11481-015-9586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hegde VL, Tomar S, Jackson A, et al. Distinct MicroRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Δ9-tetrahydrocannabinol in vivo regulation of CCAAT/enhancer-binding protein α by microRNA-690. J Biol Chem. 2013;288:36810–36826. doi: 10.1074/jbc.M113.503037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang X, Hegde VL, Rao R, et al. Histone modifications are associated with Delta(9)-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J Biol Chem. 2014;289:18707–18718. doi: 10.1074/jbc.M113.545210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Szutorisz H, Hurd YL. High times for cannabis: epigenetic imprint and its legacy on brain and behavior. Neurosci Biobehav Rev. 2018;85:93–101. doi: 10.1016/j.neubiorev.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Möhnle P, Schütz SV, Schmidt M, et al. MicroRNA-665 is involved in the regulation of the expression of the cardioprotective cannabinoid receptor CB2 in patients with severe heart failure. Biochem Biophys Res Commun. 2014;451:516–521. doi: 10.1016/j.bbrc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 179.Chandra LC, Kumar V, Torben W, et al. Chronic administration of Δ9-tetrahydrocannabinol induces intestinal anti-inflammatory microRNA expression during acute SIV infection of rhesus macaques. J Virol. 2014;89:1168–1181. doi: 10.1128/JVI.01754-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tomasiewicz HC, Jacobs MM, Wilkinson MB, et al. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol Psychiatry. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rotter A, Bayerlein K, Hansbauer M, et al. CB1 and CB2 receptor expression and promoter methylation in patients with cannabis dependence. Eur Addict Res. 2013;19:13–20. doi: 10.1159/000338642. [DOI] [PubMed] [Google Scholar]
- 182.Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40:3358–3371. doi: 10.1002/eji.201040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bronte V. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol. 2009;39:2670–2672. doi: 10.1002/eji.200939892. [DOI] [PubMed] [Google Scholar]
- 184.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mecha M, Feliú A, Machín I, et al. 2-AG limits Theiler’s virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia. 2018;66:1447–1463. doi: 10.1002/glia.23317. [DOI] [PubMed] [Google Scholar]
- 186.Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS One. 2014;9:e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thompson RF, Einstein FH. Epigenetic basis for fetal origins of age-related disease. J Womens Health (Larchmt) 2010;19:581–587. doi: 10.1089/jwh.2009.1408. [DOI] [PubMed] [Google Scholar]