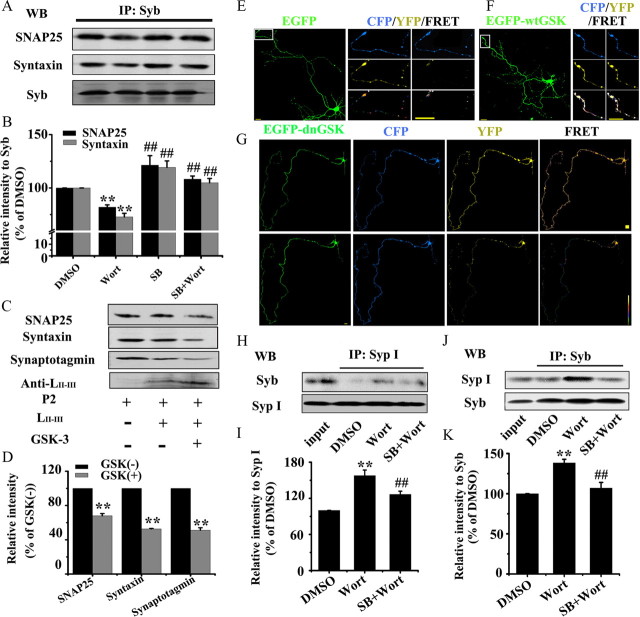
Figure 5.
A, B, GSK-3 disrupts the interactions of synaptic proteins. GSK-3 activation inhibits association of Syb with SNAP25 and syntaxin: the purified hippocampal synaptosome (P2) was incubated with DMSO, or Wort, or SB, or Wort plus SB for 30 min, then immunoprecipitated with anti-Syb and probed by anti-SNAP25, syntaxin Ia, and Syb (A) and quantitative analysis (B). Mean ± SD, ANOVA, Student's t test; **p < 0.01 versus DMSO; ##p < 0.01 versus Wort (n = 3). C, D, Phosphorylated LII-III by GSK-3β is incompetent in association with synaptotagmin, SNAP25, and syntaxin: purified LII-III peptide was phosphorylated by GSK-3β and then coimmunoprecipitated with P2 fraction for Western blotting (C) and quantitative analysis (D). E–G, GSK-3 activation hinders dissociation of Syb from SypI by FRET: the hippocampal neurons (6 DIV) were cotransfected with EGFP-labeled GSK-3β and ECFP-Syb or EYFP-SypI as indicated, then the dissociation of Syb from SypI was detected at 8 DIV. The images were recorded before (Pre) and after (Post) K+ stimulation, and the axon terminals as marked were amplified. H–K, GSK-3 activation inhibits dissociation of Syb from SypI by immunoprecipitation: the purified P2 was incubated with DMSO, Wort, or Wort plus SB for 30 min, then immunoprecipitated with anti-Syp I (H) and probed by anti-Syb, or vice versa (J), and quantitative analysis was performed (I, K). Note that GSK-3 activation disturbed the interaction of synaptic proteins during exocytosis.