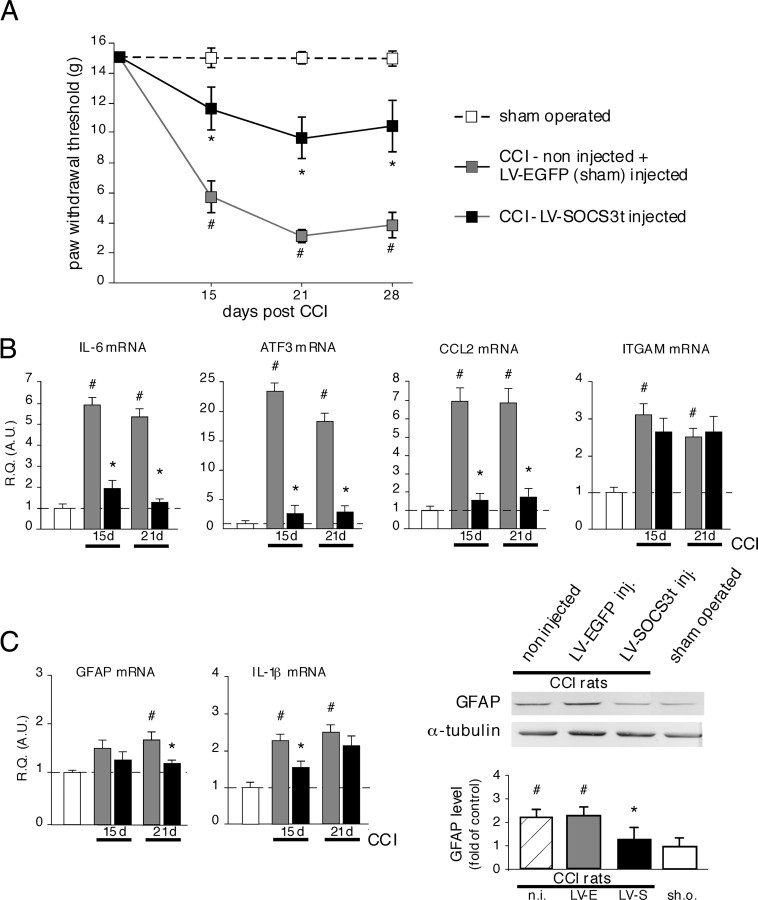
Figure 7.
Effects of LV–SOCS3t delivery into the rat dorsal spinal cord on pain behavior, local spinal cord inflammation, and glial activity. A, CCI induced mechanical allodynia in control (non-injected) or LV-EGFP-injected rats (2 μl, i.e., 70 ng of p24). This effect was not seen in sham-operated rats and was potently attenuated in LV–SOCS3t-treated rats. Each point is the mean ± SEM of n = 8–10 animals. #p < 0.001, non-injected or LV–EGFP-injected CCI rats versus sham-operated rats; *p < 0.01 LV–SOCS3t-injected CCI rats versus control or LV–EGFP-injected CCI rats. B, LV–SOCS3t-mediated blockade of JAK/STAT3 signaling in the spinal cord effectively prevented the upregulation of IL-6, CCL2, or ATF3 mRNA associated with CCI, 15 and 21 d after surgery. B, However, the high levels of ITGAM mRNA (revealing microglial activation) were unaffected by local STAT3 blockade. C, At these time points, astrocyte activation is also associated with CCI. The upregulated expression of astrocyte activity marker GFAP, observed 21 d after CCI in control rats, was attenuated in LV–SOCS3t-injected CCI animals. IL-1β, whose expression is induced after CCI, particularly in activated astrocytes, was also attenuated in LV–SOCS3t-injected CCI rats. Western blot experiments, showing low levels of GFAP protein in LVSOCS3t-injected CCI rats compared with CCI controls, further confirmed the reduced astrocyte activation in rats with locally inhibited JAK/STAT3 signaling. Data represent the mean ± SEM of n = 4–6 rats for each experimental group. #p < 0.05, non-injected or LV–EGFP-injected CCI rats versus sham operated rats; *p < 0.05, LV–SOCS3t-injected versus LV–EGFP-injected CCI rats. For Western blot experiments, each bar is the mean ± SEM of three independent experiments (n = 3 for each group). R.Q. (A.U.), Relative quantification in arbitrary units; n.i., non-injected CCI rats; LV-E, LV–EGFP-injected CCI rats; LV-S, LV–SOCS3t-injected CCI rats; sh.o., sham-operated rats.