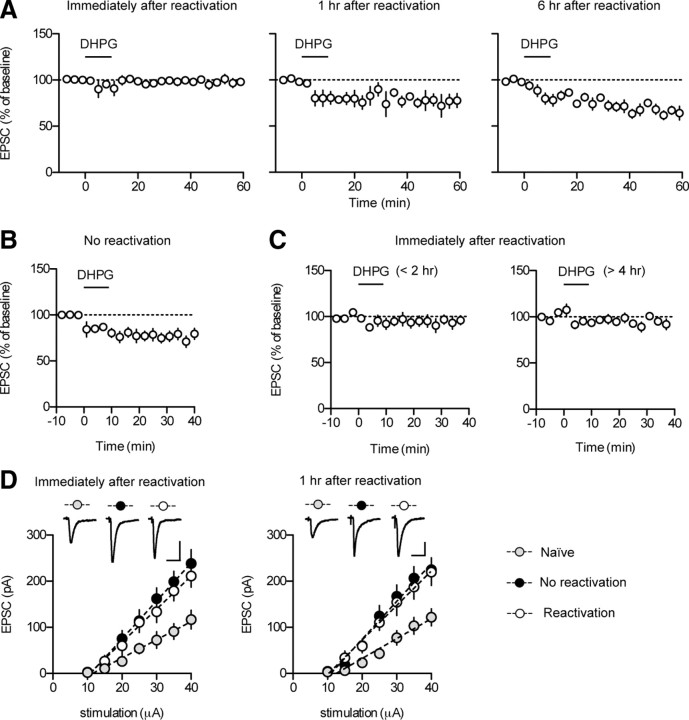
Figure 3.
Application of DHPG induces mGluRI-depotentiation at T–LA synapses in slices prepared after reconsolidation, but not before. A, DHPG application failed to induce the depotentiation in slices prepared immediately after reactivation (left). DHPG application produced a significant reduction in EPSC amplitudes in slices prepared 1 h (middle) or 6 h (right) after reactivation. B, DHPG application produced a significant reduction in EPSC amplitudes in slices prepared after exposure to the reactivation context without tone presentation (no-reactivation group). C, In slices prepared immediately after reactivation, DHPG application at various time points (<2 vs >4 h) after slice preparation does not alter the magnitude of mGluRI-depotentiation. DHPG had no discernible effects on T–LA synaptic transmission when applied either <2 or >4 h after slice preparation (<2 h after slice preparation, n = 5, 96.1 ± 5.7% of baseline, paired t test, p > 0.5; >4 h after slice preparation, n = 6, 95.2 ± 3.8% of baseline, paired t test, p > 0.2). Brain slices were prepared from conditioned animals immediately after reactivation. Whole-cell recordings were initiated at the proper times to apply DHPG at the designated time points (<2 vs >4 h). Different slices were used for each experiment. D, Input–output relationship of EPSCs between the naive, reactivation, and no-reactivation group. Amygdala slices were prepared immediately (left) or 1 h (right) after reactivation. Left, In slices prepared immediately after reactivation, synaptic efficacy was enhanced in both reactivation (open circle; n = 13; slope = 7.180 ± 0.79 pA/μA) and no-reactivation groups (filled circle; n = 14; slope = 8.224 ± 0.97 pA/μA) compared with naive controls (gray circle, n = 9, slope = 3.941 ± 0.70 pA/μA; one-way ANOVA, F(2,33) = 5.623, Newman–Keuls posttest, reactivation vs no-reactivation group, p > 0.05; reactivation vs naive group, p < 0.05; no-reactivation vs naive group, p < 0.01). Representative traces were an average of four traces from each group with an input stimulation of 35 μA. Calibration: 20 ms, 100 pA. Right, In slices prepared 1 h after reactivation, synaptic efficacy was enhanced in both reactivation (open circle; n = 9; slope, 7.484 ± 0.91 pA/μA) and no-reactivation groups (filled circle; n = 9; slope = 8.188 ± 0.97 pA/μA) compared with naive controls (gray circle; n = 7; slope, 4.311 ± 0.73 pA/μA; one-way ANOVA, F(2,22) = 4.764, Newman–Keuls posttest, reactivation vs no-reactivation group, p > 0.05; reactivation vs naive group, p < 0.05; no-reactivation vs naive group, p < 0.05). Representative traces were an average of four traces from each group with an input stimulation of 35 μA. Calibration: 20 ms, 50 pA.