Figure 1.
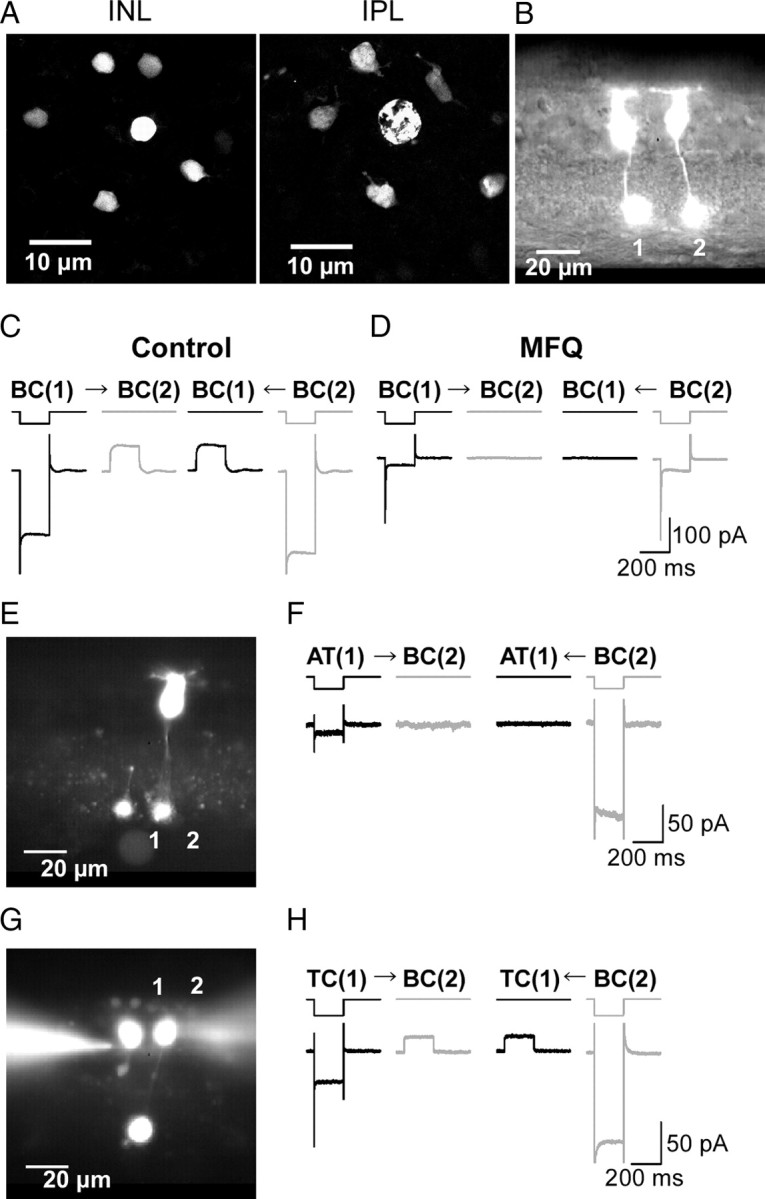
Electrical coupling between Mb1-BCs. A, Tracer coupling among Mb1-BCs. Neurobiotin was introduced through a recording pipette into the axon terminal of an Mb1-BC in the light-adapted, whole-mount preparation. After fixation, Neurobiotin was visualized by streptavidin-conjugated Alexa488, and then the preparation was observed under confocal microscope. The focal plane was at the level of the INL (left) and at the IPL (right). B, Fluorescence micrograph of two neighboring intact Mb1-BCs in the slice preparation. Each cell was filled with Lucifer yellow through a recording pipette. C, Recordings from a neighboring pair of intact Mb1-BCs [BC(1) and BC(2): different from B] in the light-adapted retina. Both cells were voltage clamped at −60 mV. Hyperpolarization (to −100 mV for 200 ms) of one cell of the pair evoked a transjunctional outward current in the other. The control external solution and the Cs+-based pipette solution were used. D, Recordings from the same pair shown in C after >30 min treatment with MFQ (10 μm). E, Fluorescence micrograph. 1, An isolated giant axon terminal of Mb1-BC [AT(1)]. 2, An intact Mb1-BC [BC(2)]. F, Recordings from the pair of AT(1) and BC(2) shown in E. Both were voltage clamped at −60 mV. Hyperpolarization (to −100 mV for 200 ms) of one cell of the pair did not evoke any response in the other cell. The control external solution and the K+-based pipette solution were used. The traces shown are from the dark-adapted condition. G, Fluorescence micrograph. 1, A truncated Mb1-BC without axon terminal [TC(1)]. 2, An intact Mb1-BC [BC(2)]. H, Recordings from the pair of TC(1) and BC(2) shown in G. Both were voltage clamped at −60 mV. Hyperpolarization (to −100 mV for 200 ms) of one cell of the pair evoked a transjunctional outward current in the other cell. The control external solution and the K+-based pipette solution were used. The traces shown are from the dark-adapted condition.
