Abstract
Experience-dependent plasticity in the cortex is often higher during short critical periods in postnatal development. The mechanisms limiting adult cortical plasticity are still unclear. Maturation of intracortical GABAergic inhibition is suggested to be crucial for the closure of the critical period for ocular dominance (OD) plasticity in the visual cortex. We find that reduction of GABAergic transmission in the adult rat visual cortex partially reactivates OD plasticity in response to monocular deprivation (MD). This is accompanied by an enhancement of activity-dependent potentiation of synaptic efficacy but not of activity-dependent depression. We also found a decrease in the expression of chondroitin sulfate proteoglycans in the visual cortex of MD animals with reduced inhibition, after the reactivation of OD plasticity. Thus, intracortical inhibition is a crucial limiting factor for the induction of experience-dependent plasticity in the adult visual cortex.
Introduction
Experience-dependent modification of cortical connections is often restricted to well defined time windows of postnatal development called critical periods (CPs) (Berardi et al., 2000, 2003; Hensch, 2005). Classical examples are the effects of altering visual experience on the developing visual cortex in mammals.
If vision in one eye is defective or made so by experimental monocular deprivation (MD) during infancy, visual acuity of that eye does not develop normally, binocular vision is impaired (Timney, 1983; Kiorpes et al., 1998; Maurer et al., 1999; Prusky et al., 2000; Prusky and Douglas, 2003), visual cortical neurons become strongly dominated by the nondeprived eye, and the proportion of binocular neurons greatly decreases (Hubel and Wiesel, 1970; Fagiolini et al., 1994). If the visual deprivation is removed early in development, the ensuing recovery is very good; on the contrary, recovery is very limited if defect removal is delayed to adulthood. Also, MD effectiveness in modifying the ocular dominance (OD) of visual cortical neurons declines with age (Hubel and Wiesel, 1970; Fagiolini et al., 1994; Hensch et al., 1998; Prusky et al., 2000; Prusky and Douglas, 2003).
The factors contributing to the decline in experience-dependent plasticity after the CPs are still poorly understood. CP closure in OD plasticity has been attributed to developmental changes in several cellular and molecular factors (Hockfield et al., 1990; Schoop et al., 1997; Hensch et al., 1998; Huang et al., 1999; Pizzorusso et al., 2002, 2006; Berardi et al., 2003; Hensch, 2005; McGee et al., 2005) and in particular to maturation of intracortical inhibition.
Intracortical inhibition is known to mature in correspondence with CP progression (Cynader and Mitchell, 1980; Mower, 1991; Fagiolini et al., 1994; Benevento et al., 1995), to be important for CP plasticity (Hensch et al., 1998; Hensch, 2005), and has been suggested to be crucial both for CP onset and closure (Hensch et al., 1998; Hanover et al., 1999; Huang et al., 1999; Fagiolini and Hensch, 2000; Rozas et al., 2001). Recently, several papers converged in indicating reduction in intracortical GABAergic inhibition as a common factor underlying plasticity restoration to the adult visual cortex (He et al., 2006, 2007; Sale et al., 2007; Maya Vetencourt et al., 2008). In particular, exposure to enriched environment or treatment with fluoxetine, which reinstates OD plasticity and promotes recovery from amblyopia in adulthood, reduces intracortical inhibition. These effects are blocked by cortical administration of diazepam, suggesting that reduction of intracortical inhibition promotes visual cortical plasticity in the adult (Sale et al., 2007; Maya Vetencourt et al., 2008).
Whether it is the mature level of cortical inhibition that limits adult cortical plasticity is, however, still unknown. If this were indeed the case, a decrease in GABAergic transmission after the normal CP end should reactivate plasticity in the adult visual cortex. We tested this hypothesis by assessing whether MD effectiveness in inducing an OD shift in the visual cortex of adult rats, normally very low after CP end (Fagiolini et al., 1994; Guire et al., 1999; Pizzorusso et al., 2002; Maya Vetencourt et al., 2008), could be restored by reducing intracortical inhibition.
Materials and Methods
Animal treatment.
Long–Evans rats older than postnatal day 85 (P85) have been used. MD and minipump implant was performed under Avertin (tribromoethanol in amylene hydrate; 1 ml/hg of body weight, i.p.) anesthesia. Minipumps (model 1007D; rate, 0.5 μl/h; Alzet) were connected to a cannula (gauge 30) implanted in the visual cortex contralateral to the deprived eye (5 mm lateral to the midline, 5 mm anterior to lambda, 4–5 mm anterior to the recording zone). For MD experiments, picrotoxin (PTX) or 3-mercaptopropionic acid (MPA) was continuously infused for 1 week in the visual cortex contralateral to the deprived eye. Animals were deprived contextually with minipump implant and recorded either after 1 week (recording during treatment) or after 4, 10, or 20 d after the end of treatment. For long-term potentiation (LTP) and long-term depression (LTD) experiments, PTX or MPA was infused in the visual cortex of undeprived animals for 4–6 d, and then slices were prepared from the treated visual cortex to assess LTP or LTD of field potentials (FPs) in layer III or IV after stimulation of the white matter.
The concentration of the drug in the minipump (100 μm) was chosen on the basis of previous in vivo and in vitro experiments (Ramoa et al., 1988; Netopilová et al., 1995; Rozas et al., 2001) and resulted in a very modest increase in spontaneous activity and peak responses, with no decrease in cell responsiveness. No sign of epileptic activity was ever seen in any of the recorded animals and their feeding, grooming, and exploratory behavior was indistinguishable from that of control animals. At a higher concentration (300 μm), the increase in spontaneous activity was more pronounced and cell responsiveness was degraded.
In vivo electrophysiology. Extracellular recordings of single-unit activity were performed essentially as previously described (Lodovichi et al., 2000; Di Cristo et al., 2001; Pizzorusso et al., 2002). Recordings were performed under urethane anesthesia (0.7 ml/hg, i.p.; 20% solution in saline). For each animal, 8–10 cells were recorded in each of at least three tracks spaced evenly (>200 μm) across the binocular primary visual cortex (Oc1B) contralateral to the deprived eye, to avoid sampling bias. Only cells with receptive fields within 20° of the vertical meridian were included in our sample. Spontaneous activity, peak response, responsiveness, and receptive field (RF) size were determined from peristimulus time histograms (PSTHs) recorded in response to computer-generated bars, averaged over at least 20 stimulus presentations (Lodovichi et al., 2000). Peak response and RF size were assessed using optimally oriented bars; responsiveness was calculated as the signal-to-noise ratio, calculating the ratio between peak response and spontaneous activity. OD was quantitatively evaluated from PSTH according to the classification of Hubel and Wiesel (1970). In addition, we calculated for each neuron a normalized OD score (Rittenhouse et al., 1999). The OD score is as follows: [(peakLE − spontLE) − (peakRE − spontRE)]/[(peakLE − spontLE) + (peakRE − spontRE)], with LE and RE representing the left and right eyes, respectively. This score is −1 for class 1 cells, +1 for class 7 cells, and 0 for class 4 cells.
For each animal, the bias of the OD distribution toward the contralateral eye was evaluated using the contralateral bias index (CBI), which is defined as CBI = {[N(1) − N(7)] + 1/2[N(2/3) − N(5/6)] + N(Tot)}/2N(Tot), where N(Tot) is the total number of recorded cells and N(i) is the number of cells in class (i). Recordings were performed blind to the treatment.
Visual evoked potentials (VEPs) were recorded as described by Di Cristo et al. (2001). VEPs were recorded by means of a micropipette (2–2.5 MΩ impedance) inserted into the binocular portion of the primary visual cortex (Oc1B). Only penetrations in which single-cell receptive fields were within 20° from the vertical meridian were used to assess VEP acuity. To record VEPs, the electrode was positioned at a depth of 450–500 μm; at this depth, VEPs had their maximal amplitude. Signals were bandpass filtered (0.1–100 Hz), amplified, and fed to a computer for analysis. Signals were averaged at least 128 events in blocks of 16 events each in synchrony with the stimulus contrast reversal. Transient VEPs in response to abrupt contrast reversal (0.5 Hz) were evaluated in the time domain by measuring the peak-to-trough amplitude and peak latency of the major negative component. Visual stimuli were horizontal sinusoidal gratings of different spatial frequency and contrast generated by a VSG2/2 card (Cambridge Research System) by custom software and presented on the face of a monitor (20 × 22 cm; luminance, 15 cd/m2) positioned 20 or 30 cm from the rat's eyes and centered on the previously determined receptive fields. Visual acuity was measured as the highest spatial frequency that still evoked a response above noise level at maximum contrast.
Assessment of GABA content and release.
GABA content was assessed by HPLC in homogenates of the binocular portion of the visual cortex in adult rats. Visual cortex samples were collected after 5–6 d from the implant of an MPA (100 μm)-filled minipump (MPA-treated cortex; N = 4 animals) or of a saline-filled minipump (control cortex; N = 4 animals).
GABA release was assessed by means of microdialysis in vivo in the recording zone of the MPA-treated cortex with respect to the corresponding region in the contralateral cortex, in nine adult animals 5–6 d after minipump implant. To implant microdialysis probes, rats were anesthetized with Avertin. After surgery, animals were returned to the cage and allowed to recover for 24 h. During the microdialysis experiment, rats were kept in plastic cages with transparent walls and were left free to move, explore the cage, groom, and eat. Microdialysis probes were continuously perfused with artificial CSF (147 mm NaCl, 1.2 mm CaCl2, 3 mm KCl) (Giovannini et al., 1997) using a microperfusion pump (1 μl/min). After 1 h of perfusion, four perfusate samples were collected at 30 min intervals; GABA was measured by HPLC analysis with fluorimetric detection, after precolumn derivatization with OPA (o-phthalaldehyde).
In vitro recordings.
Recordings in vitro were obtained using standard methods, as described by Di Cristo et al. (2001) and Sale et al. (2007). Coronal slices of visual cortex were cut and incubated (>1 h; room temperature) in cutting solution. Slices were transferred to a recording chamber superfused with the oxygenated recording solution (130 mm NaCl, 3.1 mm KCl, 1.0 mm K2HPO4, 4.0 mm NaHCO3, 5.0 mm dextrose, 1.0 mm MgCl2, 2.0 mm CaCl2, 10 mm HEPES, 1.0 mm ascorbic acid, 0.5 mm myo-inositol, 2 mm pyruvic acid, and 0.01 mm glycine, pH 7.3, 35°C). Half-maximal amplitudes of field potentials evoked from the white matter (WM) by a bipolar concentric stimulating electrode were recorded through a glass electrode filled with 3 m NaCl (1–3 MΩ) in layer III or layer IV of the binocular zone. At least 10 min of stable baseline recordings were made before theta burst stimulation (TBS) was delivered to slices to induce LTP. Stimulus intensity was adjusted to values eliciting responses approximately one-half (58.3 ± 1.1%) of the maximal response amplitude. Similar half-maximal baseline amplitudes were evoked using equivalent stimulus intensities in slices from control, MPA, or PTX experimental groups (control, 791 ± 114 μV with stimulus intensity of 1582 ± 429 μA; MPA, 671 ± 86 μV with stimulus intensity of 1115 ± 155 μA; PTX, 736 ± 67 μV with stimulus intensity of 1224 ± 117 μA). To induce LTD, low-frequency stimulation (LFS) was delivered for 15 min after recording a stable baseline for at least 15 min.
Immunohistochemistry.
Animals were anesthetized and brains were dissected and placed in ice-cold 4% paraformaldehyde in 0.1 m TBS for 24 h. Then brains were cryoprotected in 30% sucrose overnight. Forty micrometer transverse sections from the occipital cortex were cut on a sledge microtome and collected in PBS.
For the immunostaining, the procedure described by Ciucci et al. (2007) was followed. Briefly, free-floating sections were treated with blocking solution composed of 3% BSA and 20 mm lysine in PBS, pH 7.4, for 1 h. Sections were then incubated overnight at 4°C with monoclonal antibody Cat-315 (1:300 in PBS; Millipore Bioscience Research Reagents) or in a solution of biotin-conjugated lectin Wisteria floribunda (WFA) (10 μg/ml; Sigma-Aldrich). Cat-315 was then revealed with biotinylated anti-mouse IgG (1:100; Vector Laboratories), followed by Cy3-conjugated extravidin (1:500 in PBS; Sigma-Aldrich), incubated for 2 and 1 h respectively. WFA was stained with a 1 h incubation in Cy3-conjugated extravidin (1:500 in PBS; Sigma-Aldrich). Slices from MPA-treated and control animals were always processed in parallel.
Slices were coded, and confocal images (Olympus FV-300; Olympus Optical) of at least five representative fields (707 × 707 μm) for each animal were acquired (1024 × 1024 pixel) blind to the treatment using a 20× objective. All sections were acquired in random order in a single session to minimize fluctuation in laser output and degradation of fluorescence. Images were analyzed through custom-made software to count positive neurons and measure their fluorescence intensity. The code was broken only at the end of the analysis process.
Results
To test the role of intracortical inhibition in limiting adult visual cortical experience-dependent plasticity, it is critical to obtain a reduction of inhibition that obeys rather stringent constraints. Indeed, as previously proposed (Feldman, 2000), inhibition levels seem to cross two thresholds during development, the first of which corresponds to the point after which the level of inhibition is enough to allow OD plasticity to be expressed (beginning of critical period) (Fagiolini et al., 2000; Hensch, 2005). As development proceeds further, the inhibitory tone further increases and crosses a second threshold, after which the level of inhibition is enough to reduce plasticity to the low levels found in adults (closure of critical period) (Huang et al., 1999). Thus, the desired reduction should set inhibition level below the adult nonpermissive level but still above the minimum level necessary for plasticity (Netopilová et al., 1995; Hensch et al., 1998; Fagiolini and Hensch, 2000; Feldman, 2000; Hensch, 2005).
In addition, it should perturb as little as possible the visual responses of cortical neurons and should be temporally and spatially restricted to the adult visual cortex. These requirements would not be easily met by a genetic approach. Targeted pharmacology seemed to us the most suitable way to obtain the fine titration of the reduction of intracortical inhibition needed to test the hypothesis that inhibition level contributes to critical period closure.
To reduce GABAergic transmission in the adult visual cortex in vivo, we used intracortical microperfusion of MPA, a well known inhibitor of the activity of GABA synthetic enzyme GAD (glutamic acid decarboxylase), which has been previously used to reduce GABA content in nervous tissue (Netopilová et al., 1995). MPA (100 μm) was administered by means of osmotic minipumps, connected to a cannula implanted 4–5 mm anterior to the recording zone (binocular portion of the primary visual cortex) to avoid alteration of visual cortical neurons responsiveness caused by the presence of the cannula (Lodovichi et al., 2000). The advantage of this approach is that the reduction in inhibitory tone obtained is spatially restricted and its duration is easily controlled.
To estimate MPA effectiveness in reducing GABA production, we measured the GABA content in homogenates of the binocular portion of the visual cortex in adult rats. Visual cortex samples were collected after 5–6 d from the implant of a MPA-filled minipump (MPA-treated cortex, N = 4 animals) or of a saline-filled minipump (control cortex, N = 4 animals), and GABA content was assessed by means of HPLC. We found a significant reduction of GABA content in the MPA-treated cortex (t test, p < 0.001); the ratio between MPA-treated and control cortex was 0.59 ± 0.035, corresponding to a reduction in GABA production of 41%.
To estimate the effectiveness of MPA in reducing GABA release in the binocular portion of the visual cortex, we used microdialysis in vivo. The results obtained show that GABA basal release in the binocular visual cortex, assessed 5–6 d after MPA minipump implant, was significantly reduced in MPA-treated (0.51 ± 0.16 μmol/L; N = 9 animals) with respect to control, saline-treated (1.83 ± 0.12 μmol/L; N = 7 animals) cortex (t test, p < 0.001). The ratio between MPA-treated and control cortex was ∼0.3.
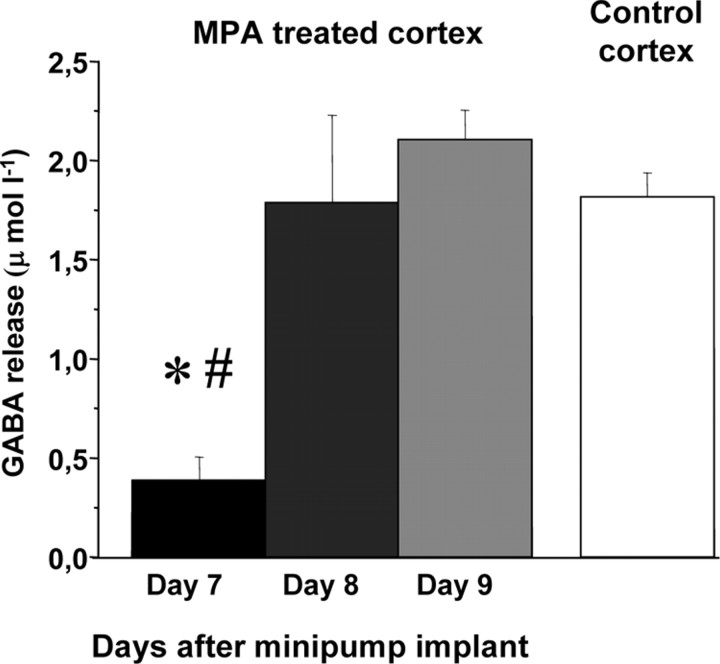
To determine when GABA release returned to normal levels after the end of MPA treatment, microdialysis was performed at day 7, day 8, and day 9 after minipump implant. Day 7 is the last day of MPA delivering for the minipumps we used (Alzet minipumps 1007D). We found (Fig. 1) that, at day 8, GABA release is significantly higher that at day 7 and does not differ from that in control animals (N = 4). The same results were found at day 9 (N = 2).
Figure 1.
Rapid recovery of basal GABA release after the end of MPA treatment. GABA release determined by in vivo microdialysis at day 7, day 8, and day 9 after minipump implant in four animals. Day 7 is the last day of minipump delivering MPA. GABA release at day 7 is significantly lower than at days 8 and 9; the latter two do not differ (one-way repeated-measures ANOVA, #p < 0.05, post hoc Holm–Sidak test). In the comparison with control cortex (N = 7 animals), only GABA release at day 7 is significantly different (one-way ANOVA, *p < 0.002, post hoc Tukey's test). Error bars represent SEM.
These results show that MPA is effective in reducing GABA release in the treated cortex and that the effects of MPA on GABA release wear off after 1 d from the end of minipump infusion.
Reduction of GABA synthesis promotes OD plasticity in the adult visual cortex
To verify whether reducing the level of intracortical inhibition could restore experience-dependent plasticity to the adult visual cortex, we tested MD effectiveness in MPA-treated rats. Adult (aged more than P85) monocularly deprived MPA-treated animals were recorded 7 d after minipump implant and MD onset. Considering the estimated duration of the minipump infusion (7 d) and the results of the microdialysis experiments, these recordings were performed when the inhibitory tone was still low.
The effectiveness of MD in shifting OD distribution was assessed by extracellular recordings of single-unit activity from the treated visual cortex contralateral to the deprived eye. Saline-treated MD rats or untreated rats of the same age were also recorded for comparison. OD of cortical neurons was objectively assessed by quantitative evaluation of responsiveness of each neuron to optimal visual stimulation of either eye. On the basis of the ratio between peak responses to stimulation of either eye, each neuron was assigned to an OD class following the classic Hubel and Wiesel criteria. In addition, to allow a finer and statistically more robust comparison of OD distributions, we computed the normalized OD score of single neurons (Rittenhouse et al., 1999).
As shown in Figure 2a, 7 d of MD were totally ineffective in shifting OD distributions in adult saline-treated rats (N = 4, 117 cells). In contrast (Fig. 2b), MD induced a significant OD shift in favor of the nondeprived eye in rats treated with MPA (N = 5, 147 cells).
Figure 2.
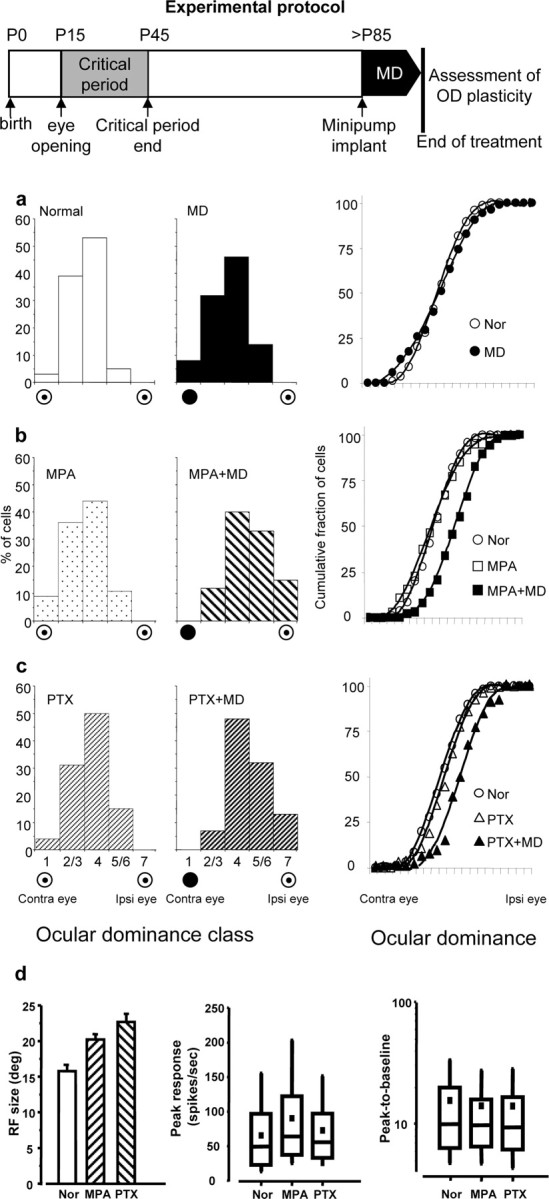
Reduction of cortical inhibition promotes OD plasticity in adult rat visual cortex. Top, Experimental protocol. a, Monocular deprivation is ineffective in adult control rats; left and middle, OD distribution for normal, nondeprived (Nor) (N = 5; 115 cells) and saline-treated, monocularly deprived adult rats (MD) (N = 4, 117 cells) do not significantly differ (χ2 test, n = 4, p > 0.05); right, same data represented as cumulative fraction of OD scores: the curve for normal animals (Nor) and control MD animals (MD) do not significantly differ [Kolmogorov–Smirnov (K-S) test, p > 0.05]. The cumulative fraction values represent the fraction of cells with an OD score less than or equal to a given OD score value. b, c, Left and middle, OD distributions for MPA-treated nondeprived (MPA) (N = 3, 90 cells); MPA-treated, monocularly deprived (MPA+MD) (N = 5, 147 cells); PTX-treated nondeprived (PTX) (N = 4, 98 cells); PTX-treated, monocularly deprived (PTX+MD) (N = 3, 91 cells) rats. Both MPA+MD and PTX+MD distributions are significantly shifted toward the nondeprived eye (ipsilateral eye) with respect to those for normal, MD, MPA, and PTX animals (p < 0.001, χ2 test; n = 4). MPA and PTX treatment without deprivation does not modify the OD distribution (χ2 test, n = 4, p > 0.05); right, same data represented as cumulative fractions of OD scores. The curves for the MPA+MD and PTX+MD groups are significantly shifted to the right (i.e., toward the nondeprived eye) with respect to those in normal and in treated nondeprived animals (K-S test, p < 0.05). Curves for undeprived MPA- or PTX-treated animals do not differ from that in normal animals (K-S test, p > 0.05). d, Functional properties of visual cortical neurons in the recorded animals. Left, Mean RF size, expressed in degrees (deg) of visual angle (±SEM); data for deprived and nondeprived MPA- or PTX-treated animals have been pooled together (no statistical difference, two-tailed t test). Normal (Nor) (115 cells), MPA-treated (MPA) (237 cells), and PTX-treated animals were recorded during the treatment (PTX) (189 cells). RFs are larger with respect to normal (one-way ANOVA, p < 0.001, Holm–Sidak post hoc test) both in MPA- and PTX-treated rats. Middle and right, Data for peak response and peak-to-baseline ratio are represented as box charts. For each box chart, the central horizontal line is the median value, and the other two horizontal lines are the 25 and 75% interquartile values; the filled square is the mean value, and the vertical bars are the 5 and 95% interquartile values. Peak response differs from normal only for MPA animals (one-way ANOVA, p < 0.05, post hoc Dunn's test); peak-to-baseline ratio (a measure of cell responsiveness) is unaffected by the treatments (one-way ANOVA, p > 0.05).
To control that reducing intracortical inhibition does not per se modify OD distribution, by simply unmasking subthreshold synaptic inputs (Sillito, 1975; Sillito et al., 1980, 1981; Ramoa et al., 1988; Jacobs and Donoghue, 1991), three adult rats were intracortically treated with MPA for 1 week but left nondeprived. Their OD distribution was completely normal (Fig. 2b).
We also assessed the effects of reducing GABAergic inhibition on cell RF size, spontaneous activity, visually evoked activity, and responsiveness. As expected, RFs were larger in MPA-treated animals (Fig. 2d), in line with the results showing that inhibition contributes to shape cortical receptive fields (Sillito, 1975; Ramoa et al., 1988; Jacobs and Donoghue, 1991). Cell responsiveness was unaffected by MPA (Fig. 2d), whereas spontaneous activity (data not shown) and peak response (Fig. 2d) were increased by less than a factor of 1.5 on the median values, and many cells in MPA-treated animals had a discharge just in the upper range of normal untreated animals.
Antagonizing GABA action on GABAA receptors promotes OD plasticity in the adult visual cortex
To strengthen the conclusion that the effects of MPA are indeed attributable to reduction of GABAergic inhibition, we used another drug, PTX, which reduces GABAergic transmission by antagonizing GABA action on its GABAA receptors, a completely different mechanism from MPA. PTX (100 μm) was administered by means of osmotic minipumps, and rats were monocularly deprived for 1 week as described for MPA.
We found that MD induced a significant OD shift in PTX-treated animals, whereas PTX treatment per se did not significantly affect OD distribution (Fig. 2c). PTX treatment significantly increased receptive field size and induced a very mild (a factor of 1.3; not significant) increase in spontaneous activity (data not shown) and peak response (Fig. 2d), with no change in responsiveness (Fig. 2d).
Together, these results show that reducing GABAergic inhibition promotes OD plasticity in the adult visual cortex.
In rats monocularly deprived during the CP, the visual deprivation causes a loss of visual acuity for the deprived eye (Fagiolini et al., 1994). We assessed visual acuity by means of visual evoked potentials recorded from the binocular portion of the primary visual cortex and found that MD, coupled with MPA (N = 3) or PTX (N = 3) treatment, significantly reduced the visual acuity of the deprived (N = 6; 0.67 ± 0.086 c/deg) with respect to the visual acuity of the undeprived eye (N = 6; 1.02 ± 0.06 c/deg); the latter was not different from the normal visual acuity of adult rats (Fagiolini et al., 1994; Sale et al., 2007).
MD performed after the end of minipump infusion is ineffective
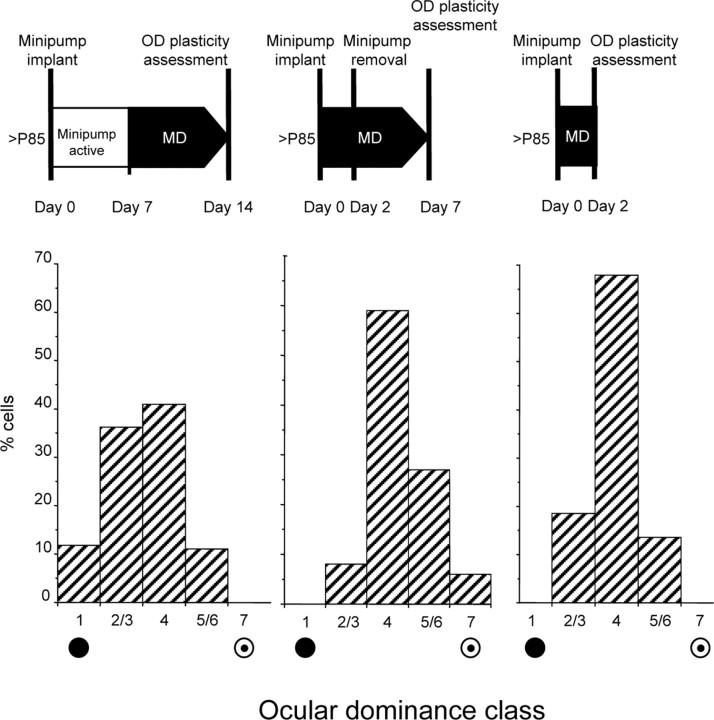
To determine whether reducing intracortical inhibition opens a window of heightened visual cortical plasticity, which is then maintained after the end of the minipump infusion, we performed MD at day 8 (day 7 is the last infusion day) and kept animals deprived for a week before assessing OD of visual cortical neurons (N = 4, 128 cells). We found no effect of MD (Fig. 3, left); indeed, the OD distribution of rats monocularly deprived after the end of minipump infusion did not differ from that of control rats (χ2 test, p = 0.339).
Figure 3.
Reduction of intracortical inhibition is necessary for the inducibility of the OD shift in response to MD but is not necessary for its maintenance. Top line, Sketch of the experimental protocols. Bottom line, OD distributions obtained with the experimental protocol shown above. Left, The OD shift is induced only if MD is performed during the period of reduced intracortical inhibition. The OD distribution in animals (N = 4, 128 cells) subjected to 7 d of MD starting from day 8 after minipump implant (PTX; pumping ends at day 7) is not significantly different from that in control undeprived rats (χ2 vs OD distribution in normals, p = 0.34). Center, The OD shift persists after the end of the treatment. OD distribution in animals (N = 4, 114 cells) with a PTX minipump implanted the same day of MD onset and disconnected 2 d later; these animals were recorded 5 d after minipump removal (total MD days = 7). The OD distribution is still significantly shifted toward the nondeprived eye (χ2 test vs normal OD distribution, p < 0.001) and is not different from the OD distribution observed in animals deprived for 7 d and recorded while inhibition was still reduced (χ2 test vs PTX MD, p = 0.8). Right, Two days of MD are sufficient to induce a detectable OD shift. OD distribution in animals (N = 3, 81 cells) recorded after 2 d of MD and reduced inhibition (PTX minipump). The OD distribution is significantly shifted toward the nondeprived eye with respect to that in normal animals (χ2 vs normal OD distribution, p = 0.003).
This result suggests that there is not a reopening of OD plasticity that outlasts the period of reduced inhibition: the enhancement in OD plasticity is strictly contingent to the presence of reduced inhibition.
OD shift persists after the end of the treatment
To assess whether MD effects, once induced, are apparent only in the presence of a reduced inhibitory tone or whether they are still evident after the end of the treatment, we recorded animals monocularly deprived for 1 week in which the minipump (filled with PTX), implanted the same day MD was performed, was disconnected 2 d after implant and MD onset. In these animals (N = 4, 114 cells), recorded 5 d after minipump removal, RF size (17 ± 0.7°) and peak response (median, 48 spikes/s; interquartile range, 25–69 spikes/s) did no longer differ from the values in normal animals (t test and Mann–Whitney test, respectively, p > 0.05). However, OD distribution was still clearly shifted toward the nondeprived eye (Fig. 3, middle) (χ2 test vs normal OD distribution, p < 0.001) and not significantly different from that observed in animals deprived for 7 d and recorded, while intracortical inhibition was still reduced (χ2 test, p = 0.8).
Similar results were obtained in animals (N = 3, 90 cells) implanted with a PTX minipump and monocularly deprived for 7 d but recorded 10 d after the end of minipump infusion (N = 3, 90 cells). The OD distribution was still significantly shifted toward the nondeprived eye with respect to that of normal adult animals (p < 0.001, χ2 test).
This shows that MD, in the presence of a reduced inhibitory level, induces changes in cortical circuits, which, once induced, do not require a low inhibitory tone to be maintained and are still present when cortical properties have returned to normality.
Two days of MD are sufficient to induce a detectable OD shift
During the rat critical period, 2 d of MD are sufficient to shift OD distribution toward the undeprived eye, although this shift is smaller than the one induced by 7 d of MD. We tested whether 2 d of MD were sufficient to do so also in adult animals with reduced inhibition. We recorded animals (N = 3, 81 cells) 2 d after minipump implant and MD onset. We found (Fig. 3, right) that a significant shift had already occurred (χ2 test vs normal OD distribution, p = 0.003) but that it was smaller than the one found after 7 d of MD.
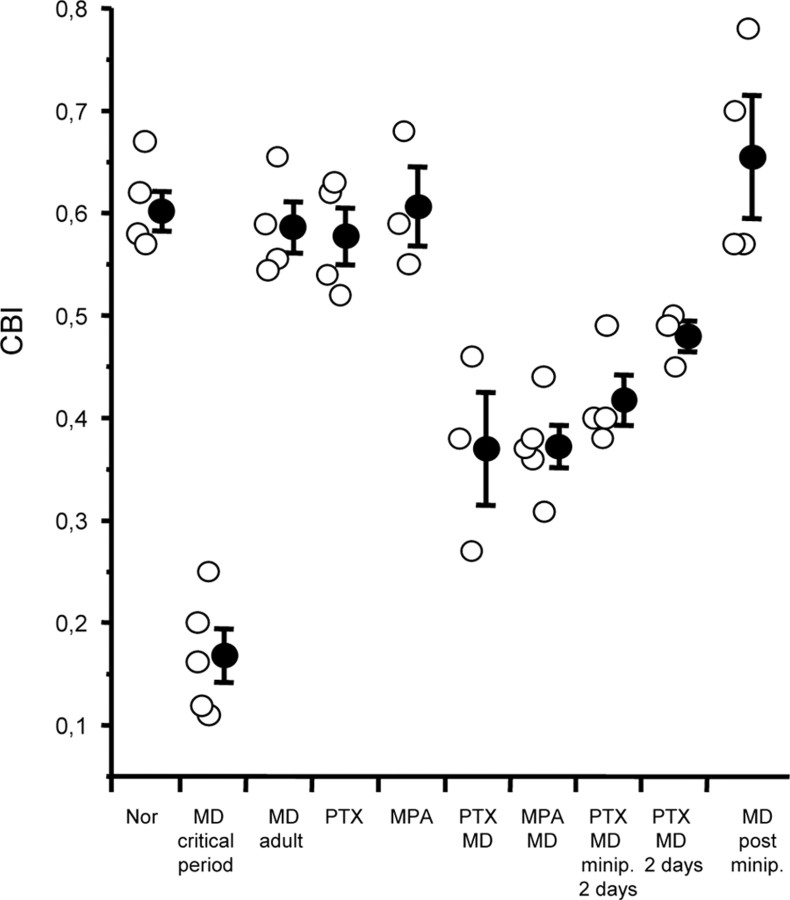
Figure 4 shows the CBI (individual values and mean ± SEM) for each experimental group; CBI for rats monocularly deprived and treated with MPA or PTX for 7 d (0.37 ± 0.02 and 0.37 ± 0.05, respectively) were significantly different from CBI in normal animals (0.6 ± 0.01), saline-treated MD animals (0.59 ± 0.02), and nondeprived MPA- or PTX-treated animals (0.61 ± 0.04 and 0.58 ± 0.02). The CBI for animals monocularly deprived for 7 d but with minipump removed after 2 d (PTX MD minipump 2 d, 0.4 ± 0.04) was also significantly different from that in normal, saline-treated MD and nondeprived MPA- or PTX-treated animals but did not differ from that in MD animals treated with MPA or PTX for 7 d. CBIs for PTX-treated animals subjected to only 2 d of MD (PTX MD 2 d, 0.48 ± 0.015) were significantly different from those in normal animals, saline-treated MD, nondeprived MPA- or PTX-treated animals, and animals subjected to 1 week MD after the end of the minipump infusion. CBIs for animals subjected to 1 week MD after the end of minipump infusion (0.66 ± 0.06) did not differ from that in normal, saline-treated MD and nondeprived MPA- or PTX-treated animals and were significantly different from those in MD animals treated with MPA or PTX for 7 d, in animals subjected to only 2 d of MD, and in animals monocularly deprived for 7 d but with minipump removed after 2 d.
Figure 4.
CBI values for all recorded animals in the different experimental groups. The open symbols represent data for single animals, and the filled symbols represent the mean CBI (±SEM) for each experimental group. CBIs for monocularly deprived rats treated with MPA or PTX significantly differ from CBIs in normal animals (Nor), saline-treated MD animals, and nondeprived MPA- or PTX-treated animals (one-way ANOVA, p < 0.001), whereas the latter four do not differ (p > 0.05). CBIs for animals monocularly deprived for 7 d but with minipump removed after 2 d (MD minipump 2 d) significantly differ from those in normal, saline-treated MD and nondeprived MPA- or PTX-treated animals but do not differ from those in MPA MD or PTX MD. CBIs for animals subjected to only 2 d of MD (MD 2 d) were significantly different from those in normal animals, saline-treated MD, nondeprived MPA- or PTX-treated animals, and animals subjected to 1 week MD after the end of the minipump infusion. CBIs for animals subjected to 1 week MD after the end of minipump infusion (MD post minipump) did not differ from those in normal, saline-treated MD and nondeprived MPA- or PTX-treated animals and were significantly different from those in monocularly deprived animals treated with MPA or PTX for 7 d, in animals subjected to only 2 d of MD, and in animals monocularly deprived for 7 d but with minipump removed after 2 d. CBIs for rats monocularly deprived during the critical period are different from those of all other groups (one-way ANOVA, p < 0.001).
Figure 4 shows also the CBI for rats monocularly deprived during the critical period for comparison. Note that the shift induced by MD in adult animals with reduced intracortical inhibition is significantly smaller than the shift induced by MD during the normal critical period.
Reduction of inhibition in vivo restores WM-evoked layer III and layer IV LTP in slices from the MPA- or PTX-treated cortex
It is believed that experience-dependent cortical plasticity involves some form of use-dependent synaptic modification. The development of inhibition has been proposed to reduce the induction of plasticity in the visual cortex by acting as a gate that filters the level and pattern of activity that layer IV, the major thalamo-recipient layer, is able to relay to supragranular layers (Huang et al., 1999; Trachtenberg et al., 2000; Rozas et al., 2001). Indeed, acute application of GABA receptor antagonists on visual cortical slices at the end of the critical period increases the probability of LTP induction (Kirkwood and Bear, 1994).
To test whether the in vivo reduction in intracortical inhibition, which is able to promote experience-dependent plasticity in the adult visual cortex, also regulates activity-dependent synaptic plasticity, we assessed whether WM-evoked layer III LTP, which is normally not inducible in adult slices (Huang et al., 1999), is inducible in slices from the cortex treated in vivo with PTX or MPA. Animals were treated as described previously and slices were prepared 4–6 d after implant. Slices prepared from the visual cortex of saline-treated animals or slices prepared from the untreated cortex of MPA- or PTX-treated animals served as control group.
As expected, LTP induction was absent in control slices from adult animals (Fig. 5a). In contrast, visual cortical slices taken from both MPA- and PTX-treated animals showed a robust potentiation of FP amplitudes after WM stimulation (Fig. 5a).
Figure 5.
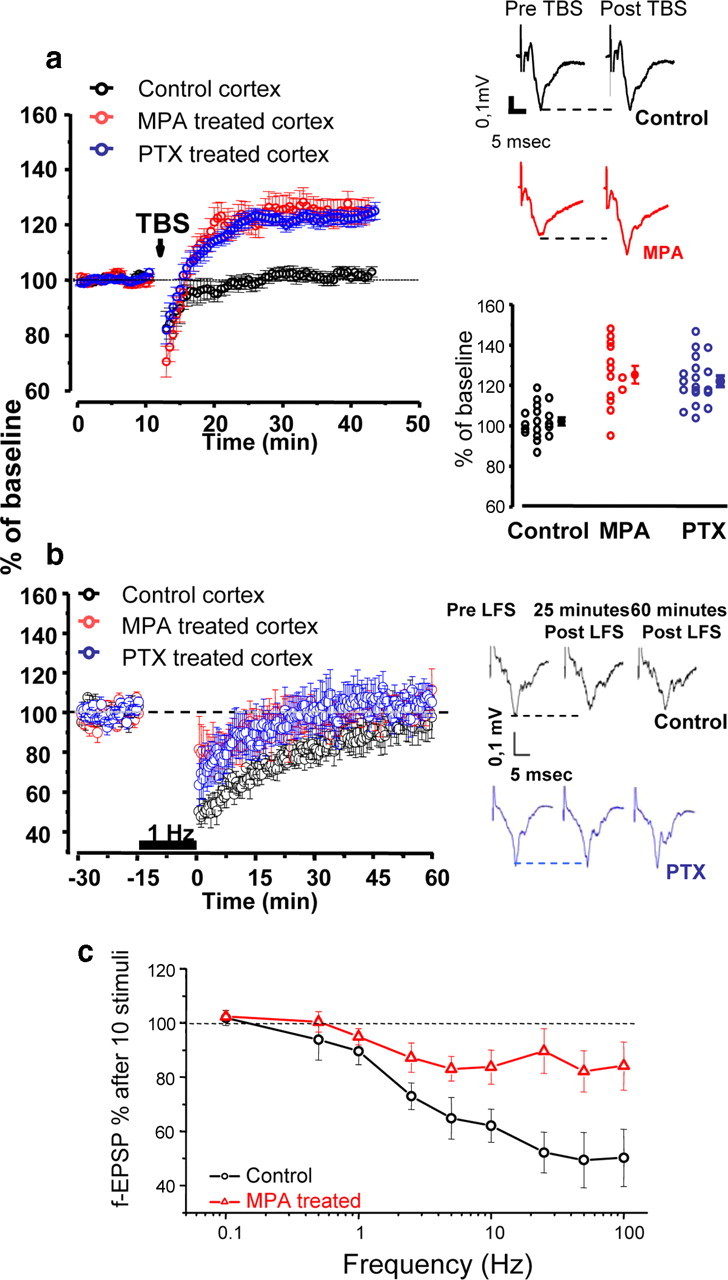
Treatment in vivo with MPA or PTX is sufficient to reactivate synaptic plasticity of the LTP type in adult visual cortex. a, Left, Average time course of layer III FP amplitude before and after TBS. The stimulating electrode was positioned at the border between WM and layer VI. Only slices from MPA (n = 13 slices, 9 animals) or PTX-treated animals (n = 19, 8 animals) show potentiation of the response after TBS. Right, Top, Average of 10 traces recorded from a control and a slice from an MPA-treated animal before and 25 min after TBS. Stimulus artifacts have been partially deleted. Only the MPA slice shows potentiation. Right, Bottom, Average and single cases of LTP in control, MPA, and PTX slices 25 min after TBS. Data for MPA and PTX are significantly potentiated with respect to control (one-way ANOVA, p < 0.001; post hoc Tukey's test MPA or PTX vs control, p < 0.05; MPA vs PTX, p > 0.05). b, Left, Average time course of layer III FP amplitude before and after LFS. The stimulating electrode was positioned at the border between WM and layer VI. Neither slices from controls (n = 11 slices, 6 animals) nor slices from MPA (n = 9 slices, 4 animals)- or PTX-treated animals (n = 10 slices, 4 animals) show depression of the response 60 min after LFS [two-way repeated-measures ANOVA, time (10 min) by condition (baseline vs 60 min after LFS)]. Right, Average of 10 traces recorded from a control and a slice from an MPA-treated animal before, and 25 and 60 min after LFS. Stimulus artifacts have been partially deleted. c, Average of layer III FP amplitude after 10 stimuli at different frequencies. The stimulating electrode was positioned at the border between WM and layer VI. MPA (n = 14 slices, 5 animals)-treated animals show less short-term depression of the response than control animals (n = 10 slices, 6 animals) (two-way repeated-measures ANOVA, p < 0.05). Error bars indicate SEM.
This would suggest that the treatment used is sufficient to attenuate the filtering action of inhibition on activity-dependent synaptic plasticity of the LTP type.
We also assessed the inducibility of LTP of white matter, layer III responses in slices from animals with reduced intracortical inhibition and subjected to previous MD for 7 d. We found that LTP was still inducible in MPA-treated MD animals (supplemental Fig. 2, available at www.jneurosci.org as supplemental material), although there was a much higher variability and the time course was somewhat different from what was found in non-MD animals. Thus, 7 d of MD do not occlude LTP inducibility in layer III of MPA-treated adult animals. This might be related to the fact that MD in adult animals with reduced inhibition does not induce a full shift of OD, as the one produced by MD during the critical period.
To assess whether the enhancement of LTP-like synaptic plasticity persisted after the end of the treatment, we assessed LTP inducibility 3–4 d after the end of minipump infusion (day 10–11 after minipump implant). We found that LTP was not inducible in slices from MPA (N = 13)- or PTX (N = 7)-treated visual cortex taken 3–4 d after the end of the treatment.
This is in line with the result that MD for 7 d performed after the end of the treatment (from day 8 after minipump implant) produces no effect on OD.
To investigate whether reducing intracortical inhibition similarly affected layer III and layer IV plasticity, we assessed LTP of layer IV responses elicited by white matter stimulation in slices from animals treated with MPA and from control, saline-treated animals. Stimulation protocol for LTP was identical with that used to assess layer III plasticity. We found that LTP was induced in slices from MPA-treated but not from control animals (supplemental Fig. 1a, available at www.jneurosci.org as supplemental material). Thus, reduction of intracortical inhibition is sufficient to enhance synaptic plasticity of the LTP type also at the level of the thalamo-recipient layer IV.
Reduction of inhibition in vivo does not enhance WM-evoked layer III and layer IV LTD in slices from MPA- or PTX-treated cortex
To determine whether the reduction in the intracortical inhibitory tone enhanced also synaptic plasticity of the LTD type, we assessed LTD inducibility in visual cortical slices during MPA or PTX treatment. Slices were prepared 5–6 d after minipump implant and LTD was assessed in layer III after white matter stimulation with a low frequency (1 Hz) for 15 min. We found (Fig. 5b) that LTD was inducible neither in control slices (11 slices, 6 animals, minipump filled with saline) nor in slices taken in MPA-treated animals (9 slices, 4 animals) or in PTX-treated animals (10 slices, 4 animals). Indeed, the amplitude of field potentials at 50–60 min after LFS had returned to baseline for all experimental groups [two-way repeated-measures ANOVA for each experimental group, control vs MPA, PTX, time (10 min, repeated factor) by condition, last 10 min of baseline vs 50–60 min after LFS, condition is a nonsignificant factor for controls, p = 0.7; MPA, p = 0.2; and PTX, p = 0. 56]. This shows that reducing GABAergic inhibition in the adult visual cortex enhances LTP- but not LTD-like plasticity.
We also assessed LTD of layer IV responses elicited by white matter stimulation in slices from animals treated with MPA and from control, saline-treated animals. Stimulation protocol for LTD was identical with that used to assess layer III LTD. We found that LTD was not inducible in either MPA or control group (supplemental Fig. 1b, available at www.jneurosci.org as supplemental material). Thus, reducing the intracortical inhibition selectively enhances the LTP type of plasticity both in layer III and in layer IV.
We observed a lower reduction of field potential amplitude in layer III in slices from the visual cortex of MPA- or PTX-treated animals with respect to controls at 20–30 min after LFS, which was just significant [two-way ANOVA, time (10 min, repeated factor) by condition, factor condition significant; control vs MPA, p = 0.026; control vs PTX, p = 0.049]. We investigated this point further in slices from animals treated with MPA using a protocol for assessing short-term dynamics of synaptic responses.
We found that the progressive reduction in field EPSP amplitude after repetitive stimulation (10 stimuli) of increasing temporal frequency found in control slices was strongly reduced in slices taken from MPA-treated animals (Fig. 5c).
These data show that reducing GABAergic inhibition in the adult reduces the depression in layer III responses to white matter stimulation evident with high-frequency stimulation and increases the capability of the cortex to relay incoming high-frequency patterns of activity to the supragranular layers.
Reactivation of OD plasticity in the adult visual cortex is associated with a reduction in chondroitin sulfate proteoglycans
Recent results have shown that degrading chondroitin sulfate proteoglycans (CSPGs) restores OD plasticity to the adult rat visual cortex (Pizzorusso et al., 2002, 2006), suggesting that the developmental increase of CSPGs is one of the factors leading to the decline of cortical plasticity. Degradation of matrix components is thought to be a crucial step in the cascade of events that leads from detection of activity changes to long-term modifications of synaptic efficacy (Baranes et al., 1998) and in particular to MD effects (Hensch et al., 1998; Oray et al., 2004); indeed, MD-induced changes in OD and in spine density are absent in tissue plasminogen activator (tPA) knock-out mice (Mataga et al., 2002, 2004).
To analyze whether the enhancement of OD plasticity in the adult visual cortex caused by MPA treatment was associated with a reduction in the expression of CSPGs, we counted WFA- and CAT-315-positive profiles in the primary visual cortex of adult rats monocularly deprived for 1 week and treated with MPA.
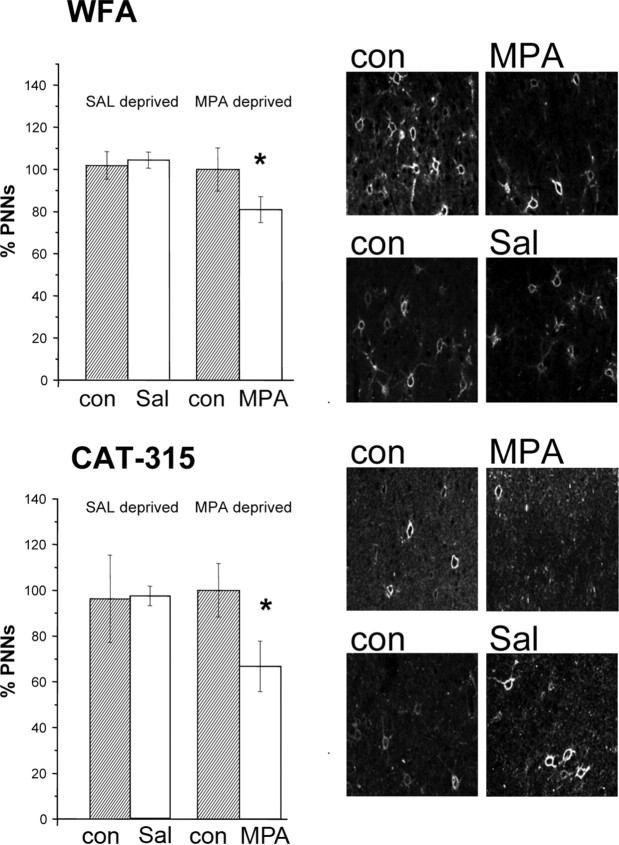
We found that the number of WFA-positive neurons (Fig. 6, top) in the MPA-treated cortex (the cortex contralateral to the deprived eye) (N = 12; mean number of cell/field, 30 ± 2.3), was lower than in the untreated cortex, ipsilateral to the deprived eye (mean number of cell/field, 37 ± 3.8; paired t test, p = 0.036). This effect was significant both in layers II–III (p = 0.029) and in layer IV (p = 0.045) but not in layers V–VI (p = 0.7). Density of WFA-positive cells in the cortex ipsilateral to the deprived eye was not different from that observed in the untreated cortex of nondeprived rats (N = 4; t test, p = 0.67). This result held for all layers.
Figure 6.
MD coupled with MPA (7 d) reduces CSPG expression in the adult visual cortex (treated cortex contralateral to the deprived eye). Top, Left, Density of WFA-positive cells in MPA and saline-treated rats. Density of WFA-positive profiles is normalized to that of the untreated cortex, ipsilateral to the deprived eye, of saline-treated animals. The cortex contralateral to the deprived eye shows a significantly lower density than the ipsilateral untreated cortex in MPA-treated rats (paired t test, *p = 0.036) but not in saline-treated rats (paired t test, p = 0.81). Error bars indicate SEM. Top, Right, Examples of WFA staining in the different experimental groups. Con, Control cortex ipsilateral to the deprived eye; MPA, MPA-treated cortex, contralateral to the deprived eye; Sal, saline-treated cortex, contralateral to the deprived eye. Bottom, Left, Density of Cat-315-positive cells in MPA and saline-treated rats. Density of Cat-315 profiles in each group is normalized to that of the untreated cortex, ipsilateral to the deprived eye, of saline-treated animals. The cortex contralateral to the deprived eye has a significantly lower density than the ipsilateral untreated cortex in MPA-treated rats (paired t test, p = 0.002) but not in saline-treated rats (paired t test, p = 0.98). Error bars indicate SEM. Bottom, Right, Examples of Cat-315 staining in the different experimental groups. Con, Control cortex ipsilateral to the deprived eye; MPA, MPA-treated cortex; Sal, saline-treated cortex. Scale bar, 65 μm.
Similar results were obtained analyzing CAT-315 staining (Fig. 6, bottom). This antibody labels the core protein of the CSPG aggrecan. In kitten visual cortex, the expression of aggrecan correlates with critical period and is regulated by visual experience (Hockfield et al., 1990; Lander et al., 1997). We found that, in MPA-treated rats (N = 12), the density of CAT-315-positive neurons (mean number of cells/field, 13.5 ± 2.3) was significantly reduced in the cortex contralateral to the deprived eye, MPA-treated, with respect to the ipsilateral untreated cortex (mean number of cells/field, 20.3 ± 2.4; paired t test, p = 0.002). Also, for CAT-315 staining, the density of positive cells in the cortex ipsilateral to the deprived eye was not different from that observed in the untreated cortex of nondeprived rats (t test, p = 0.98).
Both for WFA and CAT-315 staining, no significant difference between the two cortices was present in the control, saline-treated monocularly deprived animals (N = 4; paired t test, p = 0.81 for WFA, p = 0.88 for CAT-315) (Fig. 6). Thus, monocular deprivation per se does not cause a reduction of CSPG positive neurons in the cortex contralateral to the deprived eye; a reduction is found only if MD is coupled with MPA treatment.
To control whether CSPG density was reduced also when OD plasticity was enhanced by antagonization of GABAA receptors, we counted WFA- and CAT-315-positive profiles in the primary visual cortex of adult rats treated with PTX or saline and monocularly deprived for 1 week. We found that PTX treatment produced the same results as MPA treatment (supplemental Fig. 3a, available at www.jneurosci.org as supplemental material).
Next, we asked whether the reduction of inhibitory transmission was sufficient to reduce CSPG-positive neurons in the absence of monocular deprivation. To address this point, we quantified the number of WFA- and CAT-315-positive cells in the visual cortex of nondeprived rats treated with MPA (N = 5). We found no difference between the MPA-treated and the untreated cortex both for WFA (MPA-treated cortex, 54.2 ± 4 cells/field; control cortex, 54.6 ± 3.6 cells/field; paired t test, p = 0.916) and for CAT-315 (MPA-treated cortex, 10.7 ± 1.6 cells/field; control cortex, 7.5 ± 0.7 cells/field; paired t test, p = 0.101).
These data show that MPA treatment per se does not affect CPSG expression but that the reduction of CPSG-positive cells occurs only if a long-term plasticity process is made possible by the simultaneous presence of an imbalance in visual experience between the two eyes and a reduction of the inhibitory tone.
Interestingly, we found that, after 2 d of MD and reduction of inhibition, no effect on WFA or CAT315 nets could be detected (supplemental Fig. 3b, available at www.jneurosci.org as supplemental material), whereas an OD shift was already observed. This suggests that changes in expression of CSPG markers follow the first expression of plasticity.
Discussion
We have shown that, by reducing intracortical inhibition, it is possible to partially reactivate OD plasticity in the visual cortex of adult rats. This suggests that the adult level of inhibition actively restricts cortical plasticity.
The present results are the first direct demonstration that, in normal adult animals, in which inhibitory processes have completed their development, a brief reduction of GABAergic inhibition is sufficient to reopen a window of plasticity in the visual cortex well after the normal closure of the critical period.
These results support the conclusion drawn by two recent papers that the enhancement of visual cortical plasticity obtained in adult rats by environmental enrichment (Sale et al., 2007) or fluoxetine treatment (Maya Vetencourt et al., 2008) is attributable to the reduction of intracortical inhibitory tone. Interestingly, the reduction in GABA release found in our MPA-treated animals is comparable with that found by Sale et al. (2007) in enriched animals.
In addition, our results support the hypothesis that the development of inhibition contributes to critical period closure, in accordance with the findings by Huang et al. (1999) that BDNF overexpression accelerates the development of inhibitory circuitry and the closure of the critical period. They are also in agreement with those by Fagiolini and Hensch (2000), who observed, using long-term MD as a probe for OD plasticity, that the critical period is prolonged into adulthood in mice knocked out for the enzyme responsible for GABA production.
We underline the fact that the reduction of intracortical inhibitory tone that promotes adult OD plasticity increases, as expected, spontaneous activity and the amplitude of the peak discharge in response to visual stimulation but does not affect cell responsiveness to visual stimulation (calculated as the peak signal-to-noise ratio). This suggests that, although pharmacological in nature, the manipulation of intracortical inhibition we performed does not disrupt cortical function, a suggestion reinforced by the observation that the same amount of GABA release reduction we obtained with MPA was achieved by Sale et al. (2007) with a physiological manipulation, namely, exposure to environmental enrichment.
Previous studies have shown that acute reduction of the inhibitory tone by a GABA antagonist reveals the presence of subthreshold synaptic inputs to cortical neurons (Sillito, 1975; Ramoa et al., 1988; Jacobs and Donoghue, 1991) and, in particular, of inputs from the nondominant eye. The OD change is transient, being lost after the end of the drug application (Sillito et al., 1980, 1981). Therefore, we have controlled that reducing intracortical inhibition did not per se modify OD distribution by simply unmasking subthreshold synaptic inputs. Two experimental findings suggest that indeed this is not the case. First, the OD distribution after 7 d of treatment with MPA or PTX is completely normal in non-MD animals. Second, the OD shift is still evident after the end of drug infusion, when cell spontaneous activity, peak response, and receptive field size have returned to normal. This suggests that the OD shift toward the ipsilateral, nondeprived eye found when MD is coupled with reduced inhibitory tone is attributable to long-term plastic changes in the OD of visual cortical neurons. We cannot, however, exclude that the acute unmasking of ipsilateral subthreshold connections plays a role in facilitating the onset of long-term OD plastic changes in the presence of monocular deprivation.
OD shift is induced only if MD is performed during the period of reduced inhibition, but, once induced, it persists after the end of the pharmacological treatment used to reduce inhibition; these results suggest that the inhibitory tone controls plasticity induction rather than maintenance. In other words, the OD of visual cortical neurons cannot be easily modified by MD in adult rats under condition of adult levels of intracortical inhibition; however, once its modification is reenabled by the lowered inhibitory tone, its maintenance is not directly dependent on the level of inhibitory tone in the cortex. In line with these results is the observation that 2 d of reduced inhibition are sufficient to allow a small but significant shift in OD. These findings again suggest that the gating action exerted by the adult levels of inhibition acts on the probability of induction of plastic changes.
The results obtained in the slice experiments show that reducing inhibition in the adult visual cortex enhances the induction of activity-dependent long-term potentiation of synaptic efficacy, but not activity-dependent long-term depression, both in layer IV and in layer III, and reduces short-term depression in layer III responses, after white matter stimulation. These results strengthen the hypothesis that reducing inhibition promotes adult visual cortical plasticity by increasing the capability of the cortex to relay incoming high-frequency patterns of activity to the supragranular layers (Kirkwood and Bear, 1994; Rozas et al., 2001) but also show that LTP-type synaptic plasticity is enhanced at the level of the thalamo-recipient layer IV.
These modifications, together with the increased excitability of cortical neurons caused by the reduction of GABA activity, would facilitate a potentiation of the input from the undeprived eye.
The increase in activity caused by the reduction of the inhibitory tone might also activate homeostatic feedback mechanisms in visual cortical network (Kaneko et al., 2008; Leamey et al., 2009) leading to a downward scaling of synaptic activity. Homeostatic mechanisms are considered to be non-eye specific and therefore could also affect deprived eye responses, reducing the chances of surpassing the threshold for activating visual cortical neurons. This process might be particularly present in neurons in which the potentiation of the nondeprived eye inputs maintains a high level of neuronal activity and could contribute to the degradation of the receptive field properties of visual cortical neurons and the reduction in the visual acuity of the deprived eye.
Another factor affecting deprived eye acuity could be Otx2 signaling. It has been recently demonstrated that this homeoprotein controls maturation of cortical parvalbumin-positive GABAergic interneurons and promotes perineuronal net (PNN) formation; Otx2 is transported from the retina to the visual cortex in an activity-dependent way, and it contributes to the regulation of visual cortex critical period plasticity (Sugiyama et al., 2008). Our results show that the expression of OD plasticity in the adult visual cortex of MD animals with reduced intracortical inhibition is followed by a small reduction in PNNs, an extracellular structure surrounding, and promoting Otx2 entry into, parvalbumin-positive interneurons (Sugiyama et al., 2008). The reduction in PNN density we observed could cause a decrease in Otx2 availability to inhibitory interneurons, and this effect could be particularly relevant for those interneurons that had a strong input from the deprived eye. The decrease in Otx2 signaling could shift these set of interneurons toward a more juvenile phenotype, contributing to the reduction of visual acuity.
The reduction in PNN density observed in MD animals with reduced intracortical inhibition is not simply secondary to changes in cortical activity, either caused by MD or by the reduction in inhibitory tone, because it is not found in saline-treated MD animals or in MPA- or PTX-treated non-MD animals. This suggests that PNN degradation is a downstream step in the plasticity process in response to MD reenabled by the reduced inhibition. Degradation of extracellular matrix components by proteases such as tPA is indeed thought to be a crucial, downstream step in the cascade of events leading to MD effects during the critical period (Mataga et al., 2002, 2004; Oray et al., 2004; Hensch, 2005). We think that the reduction in PNN density observed in MPA- or PTX-treated animals after 7 d of MD is an indication that the reactivation of OD plasticity caused by reducing the inhibitory tone sets in motion endogenous mechanisms of extracellular matrix remodeling (Hensch, 2005). Consistent with these results, Sale et al. (2007) found a reduction in PNN density in animals subjected to reverse suture and exposed to enriched environment, which show a reduced inhibitory tone and recover from amblyopia, but not in control, nonenriched, reverse sutured rats, in which no reduction in inhibitory tone, no plastic changes in OD, nor recovery of visual acuity are observed.
We found that 2 d of MD and reduced inhibition do not result in detectable changes in PNN density. This suggests that PNN remodeling follows the earliest changes in OD and may contribute to phenomena of maintenance and consolidation. However, we cannot exclude that the earliest OD changes rely on small, local remodeling of PNN or of diffuse extracellular matrix (Mataga et al., 2004; Oray et al., 2004), which went undetected in our count of PNN surrounded neurons or that a reduction in PNN components or anchoring proteins takes place rapidly after MD onset but becomes visible only later, because of the slow turnover of PNNs.
The OD shift induced by MD in our adult animals with reduced intracortical inhibition is smaller than that induced during the CP. This suggests that other mechanisms that restrain the expression of OD plasticity are still in place. The reduction in PNN density we found is partial; therefore, PNN density remains higher than that during the peak of the critical period (Pizzorusso et al., 2002). Indeed, in MD MPA-treated animals, we found a PNN density (WFA staining) of ∼80% of the adult value, whereas PNN density in normal P22 and P35 rats is ∼40 and 65% of the adult value. In addition, the presence of factors limiting chromatin remodeling (Putignano et al., 2007), of signaling from extracellular factors, for example from myelin proteins on Nogo receptors (McGee et al., 2005; Fagiolini et al., 2009), as well as the characteristic of adhesion molecules (Hensch, 2005), might contribute to partially restrain the full expression of long-term OD changes in response to MD in animals with reduced intracortical inhibition. It will be important to investigate which genes show changes in expression levels in adult animals monocularly deprived and with reduced intracortical inhibition. These genes might belong to the gene set activated by experience also in adults and not only during the critical period (“common gene set”) (Majdan and Shatz, 2006); this would indicate that reducing intracortical inhibition acts by potentiating factors already at work in adult visual cortical plasticity (Heynen and Bear, 2001; Sawtell et al., 2003). However, if these genes belong to the gene set activated by experience only during the critical period (“age-specific set”) (Majdan and Shatz, 2006), then this would indicate that reducing intracortical inhibition induces a reactivation of the same mechanisms at work during development.
In conclusion, intracortical inhibition is a crucial factor that actively limits the induction of experience-dependent plasticity in adult visual cortex. This could have implications also for brain repair, making intracortical inhibition a target for behavioral or pharmacological interventions after brain lesions. A change in inhibitory tone has indeed been found in perilesional regions in the visual cortex and also in patients with stroke in the motor cortex (Liepert, 2006). Harnessing these changes might favor functional remapping, thereby promoting functional recovery.
Footnotes
This work was supported by Italian Ministry of the University and Scientific and Technological Research COFIN and European Union projects PLASTICISE and EUROVISION.
References
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS. gamma-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res. 1995;689:172–182. doi: 10.1016/0006-8993(95)00553-3. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Ciucci F, Putignano E, Baroncelli L, Landi S, Berardi N, Maffei L. Insulin-like growth factor 1 (IGF-1) mediates the effects of enriched environment (EE) on visual cortical development. PLoS One. 2007;2:e475. doi: 10.1371/journal.pone.0000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto GM, Maffei L. Requirement of ERK activation for visual cortical plasticity. Science. 2001;292:2337–2340. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Inhibition and plasticity. Nat Neurosci. 2000;3:303–304. doi: 10.1038/73849. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Giovannelli L, Bianchi L, Kalfin R, Pepeu G. Glutamatergic modulation of cortical acetylcholine release in the rat: a combined in vivo microdialysis, retrograde tracing and immunohistochemical study. Eur J Neurosci. 1997;9:1678–1689. doi: 10.1111/j.1460-9568.1997.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Guire ES, Lickey ME, Gordon B. Critical period for the monocular deprivation effect in rats: assessment with sweep visually evoked potentials. J Neurophysiol. 1999;81:121–128. doi: 10.1152/jn.1999.81.1.121. [DOI] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19(RC40):1–5. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J Neurosci. 2001;21:9801–9813. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–514. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O'Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998;18:6411–6424. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander C, Kind P, Maleski M, Hockfield S. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J Neurosci. 1997;17:1928–1939. doi: 10.1523/JNEUROSCI.17-06-01928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamey CA, Van Wart A, Sur M. Intrinsic patterning and experience-dependent mechanisms that generate eye-specific projections and binocular circuits in the visual pathway. Curr Opin Neurobiol. 2009;19:181–187. doi: 10.1016/j.conb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006;19:41–47. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: same or different? J Neurosci. 2000;20:2155–2165. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci. 2006;9:650–659. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- Mataga N, Nagai N, Hensch TK. Permissive proteolytic activity for visual cortical plasticity. Proc Natl Acad Sci U S A. 2002;99:7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44:1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Maurer D, Lewis TL, Brent HP, Levin AV. Rapid improvement in the acuity of infants after visual input. Science. 1999;286:108–110. doi: 10.1126/science.286.5437.108. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Netopilová M, Drsata J, Kubová H, Mares P. Differences between immature and adult rats in brain glutamate decarboxylase inhibition by 3-mercaptopropionic acid. Epilepsy Res. 1995;20:179–184. doi: 10.1016/0920-1211(94)00068-8. [DOI] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci. 2003;17:167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Experience-dependent plasticity of visual acuity in rats. Eur J Neurosci. 2000;12:3781–3786. doi: 10.1046/j.1460-9568.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron [Erratum (2007) 54:177] 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Paradiso MA, Freeman RD. Blockade of intracortical inhibition in kitten striate cortex: effects on receptive field properties and associated loss of ocular dominance plasticity. Exp Brain Res. 1988;73:285–296. doi: 10.1007/BF00248220. [DOI] [PubMed] [Google Scholar]
- Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- Rozas C, Frank H, Heynen AJ, Morales B, Bear MF, Kirkwood A. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci. 2001;21:6791–6801. doi: 10.1523/JNEUROSCI.21-17-06791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Schoop VM, Gardziella S, Müller CM. Critical period-dependent reduction of the permissiveness of cat visual cortex tissue for neuronal adhesion and neurite growth. Eur J Neurosci. 1997;9:1911–1922. doi: 10.1111/j.1460-9568.1997.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Patel H. Inhibitory interactions contributing to the ocular dominance of monocularly dominated cells in the normal cat striate cortex. Exp Brain Res. 1980;41:1–10. doi: 10.1007/BF00236673. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Blakemore C. The role of GABAergic inhibition in the cortical effects of monocular deprivation. Nature. 1981;291:318–320. doi: 10.1038/291318a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Timney B. The effects of early and late monocular deprivation on binocular depth perception in cats. Brain Res. 1983;283:235–243. doi: 10.1016/0165-3806(83)90180-3. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 2000;287:2029–2032. doi: 10.1126/science.287.5460.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]