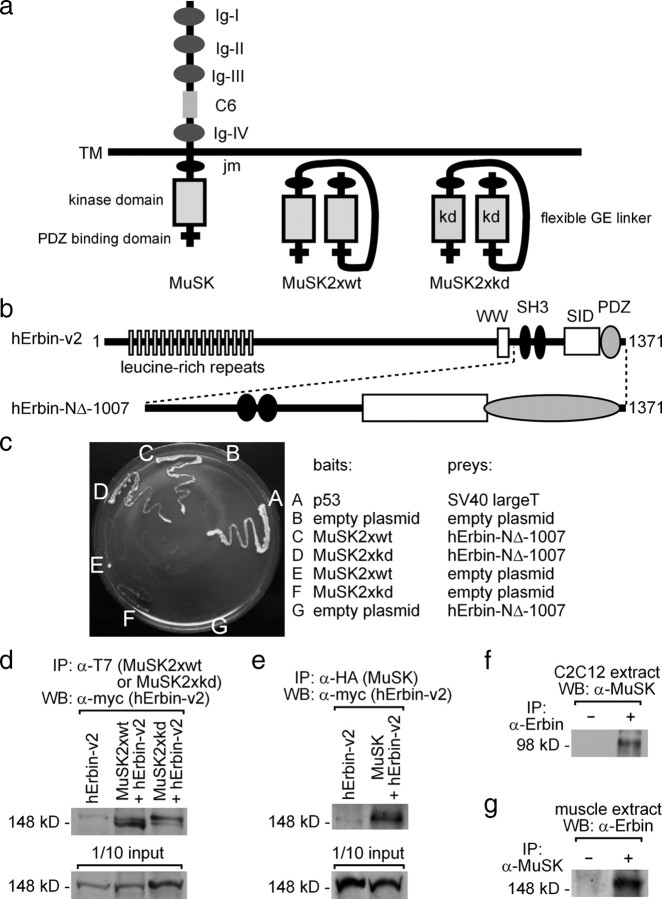
Figure 1.
Erbin interacts with MuSK. a, Scheme of wild-type MuSK and two MuSK mutants (MuSK2xwt, MuSK2xkd). The mutants are generated by linking two intracellular parts of MuSK together via a peptide linker, thereby imitating the dimeric active intracellular part of wild-type MuSK. Each of the mutants was used for yeast two-hybrid. The term kd reflects the use of a kinase-defective MuSK mutant. Ig, Ig-like domain; C6, cysteine-rich region; TM, transmembrane segment; JM, juxtamembrane region. b, Illustration of the epitopes of the human Erbin-variant2 (hErbin-v2) (Favre et al., 2001). Below the scheme of human Erbin-v2, the area of Erbin interacting with MuSK is drawn (hErbin-NΔ-1007). SID, Smad-interacting domain; WW, Trp-Trp; SH3, Src homology domain 3. c, The growth of different yeast clones on agar lacking histidine and adenine is shown. Note that yeast cells are able to grow if they contain either MuSK2xwt or MuSK2xkd as bait plasmids and the C-terminal part of human Erbin (hErbin-NΔ-1007) as prey plasmid, or if they contain p53 and SV40 large T antigen (positive control). d, e, Extracts of transiently transfected HEK293 cells were used to precipitate MuSK2xwt, MuSK2xkd, or full-length hemagglutinin (HA)-tagged MuSK. As a consequence human Erbin-v2 was coprecipitated. The amount corresponding to 1/10 of the input was ascertained by Western blot. f, g, Precipitation of endogenous Erbin or MuSK proteins from C2C12 cells or hindlimb muscle cell extracts coprecipitated MuSK or Erbin as indicated.