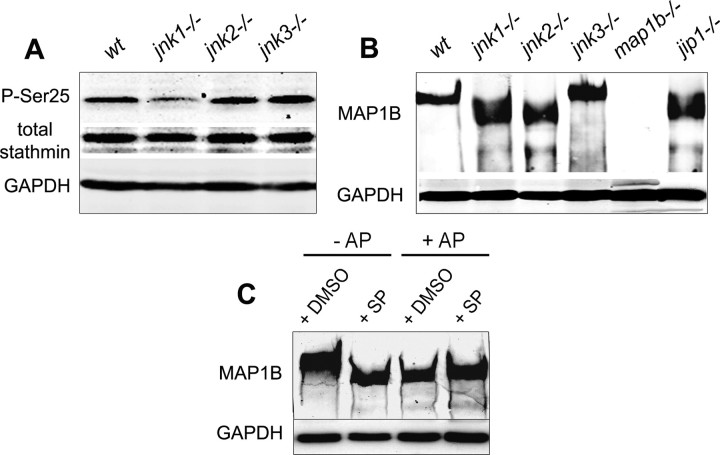
Figure 6.
Regulation of stathmin and MAP1B phosphorylation by JNKs: Western blot analysis with GADPH used as internal loading control. A, Relative amounts of total stathmin and stathmin phosphorylated on serine 25 (P-Ser25) in lysates of wild-type and JNK-deficient DRG neurons cultured for 48 h; note the decrease in stathmin phosphorylation in regenerating jnk1−/− neurons. B, MAP1B staining of protein extracts from wild-type and JNK-deficient DRG neurons cultured for 48 h, showing a shift in the protein band representing MAP1B from higher to lower apparent molecular weight for jnk1−/− and jnk2−/−, but not jnk3−/− neurons. This shift reflects a reduced level of MAP1B phosphorylation as demonstrated in C, MAP1B in lysates of N2A neuroblastoma cells cultured for 48 h is phosphorylated under control conditions (lane 1); experimental dephosphorylation by AP treatment of lysates (lanes 3, 4) results in the same shift to a lower molecular weight as JNK inhibition by SP600125 (SP) addition to the N2A cultures (lane 2).