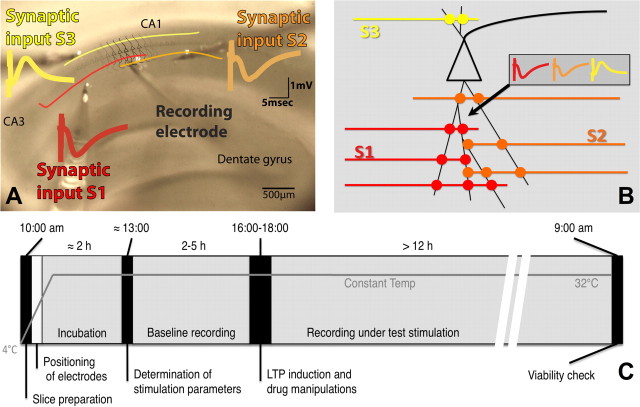
Figure 1.
Experimental protocols. A, The slice preparation with superimposed labels depicting the positioning of the electrodes. Color coding matches that used to identify the respective pathways throughout text. B, Schematic representation depicting the independent but convergent inputs onto pyramidal cells in the CA1 used in these experiments. The recording electrode placed in the stratum radiatum of CA1 records three independent field EPSPs elicited by the activation of different populations of synapses onto the same cells. C, Experimental protocol showing approximate phase lengths. Briefly, slices are cut in ice-cold aCSF and transferred to the recording chamber in which the electrodes are placed in position. Two hours of incubation allow the temperature to equalize to 32°C before input–output curves and paired-pulse stimulation tests are run to assess the optimal intensity of stimulation and confirm pathway independence, respectively. More than 2 h are still allowed to pass while baseline recordings are obtained before any drug or electrophysiological manipulations are introduced. After that, the setup returns to test-stimulation frequencies, and the experiment is allowed to develop overnight. An assessment of the viability of the slice is run the following morning (for more detailed information, see Materials and Methods).