Abstract
Risky decision making on the Iowa Gambling Task (IGT) has been observed in several psychiatric disorders, including substance abuse, schizophrenia, and pathological gambling. Such deficits are often attributed to impaired processing within the orbitofrontal cortex (OFC) because patients with damage to this area or to the amygdala, which is strongly interconnected with the OFC, can likewise show enhanced choice of high-risk options. However, whether damage to the OFC or amygdala impairs subjects' ability to learn the task, or actually affects the decision-making process itself, is currently unclear. To address these issues, rats were trained to perform a rodent gambling task (rGT) either before or after bilateral excitotoxic lesions to the basolateral amygdala (BLA) or OFC. Maximum profits in both the rGT and IGT are obtained by favoring smaller rewards associated with lower penalties, and avoiding the tempting, yet ultimately disadvantageous, large reward options. Lesions of the OFC or BLA made before task acquisition initially impaired animals' ability to determine the optimal strategy, but did not disrupt decision making in the long term. In contrast, lesions of the BLA, but not the OFC, made after the task had been acquired increased risky choice. These results suggest that, although both regions contribute to the development of appropriate choice behavior under risk, the BLA maintains a more fundamental role in guiding these decisions. The maladaptive choice pattern observed on the IGT in patients with OFC lesions could therefore partially reflect a learning deficit, whereas amygdala damage may give rise to a more robust decision-making impairment.
Introduction
Clinical and preclinical data suggest that the orbitofrontal cortex (OFC) and basolateral amygdala (BLA) are critically important in mediating cost-benefit decision making (Holland and Gallagher, 2004; Floresco et al., 2008). Although both the BLA and OFC share many reciprocal connections, contradictory reports regarding the effects of damage to these regions has lead to ambiguity concerning their precise contribution to this multifaceted process.
A well known and highly influential test used to assess real-world decision making is the Iowa Gambling Task (IGT) (Bechara et al., 1994), in which people pick from decks of cards that vary in both the size and probability of points won or lost. Patients with damage to the amygdala or ventromedial prefrontal cortex (VMPFC), which encompasses the OFC, are impaired on the IGT—choosing more cards from decks associated with larger rewards, yet disproportionately greater long-term losses (Bechara et al., 1999). Based on these original reports, IGT impairments in psychiatric populations are often interpreted as a direct indication of OFC dysfunction without any further proof aside from the behavioral impairment (Franken et al., 2008; Starcke et al., 2010). However, the role of the OFC in regulating this type of decision making has recently been challenged (Fellows and Farah, 2005; Fellows, 2007).
In the original IGT the decks are stacked, such that cards near the top of the decks lead to gains rather than losses. Therefore, an initial preference for the large reward decks occurs. Continued selection from these decks, however, yields heavier losses. Thus, the optimal strategy is to switch preference toward decks yielding smaller rewards but also smaller losses to gain more points in the long-term (Bechara et al., 1994). However, when the order of wins and losses is randomized, patients with VMPFC lesions perform similarly to controls (Fellows and Farah, 2005). Therefore, the deficit originally observed following VMPFC damage may reflect difficulties in switching strategies or perseveration, rather than a true decision-making impairment (Fellows, 2007).
However, many discrepancies arise when comparing studies using human patients. The exact location and size of the lesion, patient history, and task design all differ between studies, and could be factors in interpreting these results. For instance, patients with very discrete lesions of the OFC chose optimally on the traditional IGT (Manes et al., 2002), yet VMPFC lesion patients performed poorly on the Cambridge Gamble Task, another commonly used test of risky decision making (Clark et al., 2008). It is also unclear whether such deficits arise from a subject's inability to learn the task's rules, or whether damage to the amygdala or OFC impairs the actual decision-making process. Such questions could be resolved by comparing the consequences of lesions incurred before or after task acquisition. However, these experiments are impossible to perform in human subjects.
With the aim of resolving these issues, the current study assessed the effects of bilateral OFC or BLA lesions performed either before or after rats were trained on the rat gambling task (rGT) (Zeeb et al., 2009), a rodent decision-making paradigm comparable to the IGT.
Materials and Methods
Subjects
Subjects were male Long–Evans rats (n = 72) and male Lister Hooded rats (n = 24; all rats from Charles River Laboratories). All animals weighed 300–325 g at the start of the experiment. Animals were housed in a temperature-controlled colony room in pairs under a 12 h reverse light cycle (lights off at 8:00 A.M.). Testing took place between 9:00 A.M. and 5:00 P.M., five to six days per week. Water was available ad libitum. Animals were food restricted to ∼85% of their free-feeding weight and maintained on 14 g of standard rat chow per day, available immediately after behavioral testing. All experiments were performed in accordance with UK Animals (Scientific Procedures) Act 1986 or the Canadian Council of Animal Care, and experimental protocols were approved by the Animal Care Committee of the University of British Columbia.
Surgery
Subjects were divided into approximately equal groups, and if already tested on the rGT, were matched for baseline performance. Subjects either received bilateral lesions of the OFC (n = 26) or BLA (n = 32), or sham surgeries during which vehicle was infused into either the OFC (n = 18) or BLA (n = 20). Animals were anesthetized with 100 ml/kg Avertin [10 g of 2,2,2-tribromoethanol dissolved in 5 ml of 2-methyl-2-butanol (both from Sigma-Aldrich) and diluted in a solution of 40 ml of ethanol and 450 ml of PBS] and then secured in a stereotaxic frame with the incisor bar set at −3.3 for a flat skull. Lesions were performed by infusing the excitotoxin quinolinic acid (Sigma-Aldrich) into the area of interest. A 0.09 m solution of quinolinic acid was made fresh daily by dissolving the acid in 0.1 m PBS. The pH was then adjusted with 0.1 m NaOH to between 6.4 and 6.8. Infusions of the toxin or vehicle were accomplished using a 31 gauge stainless steel injector (Small Parts) attached to a 26 s gauge 10 μl syringe (Hamilton Company) by polyethylene tubing (Instech Laboratories). The toxin or vehicle was infused using a microinfusion pump (Harvard Apparatus). The location of infusion sites and rates of infusions were based on previous studies (Winstanley et al., 2004) and modified according to pilot data. The coordinates (Paxinos and Watson, 1998) and rates of infusion for BLA lesions were as follows: site 1: anterior–posterior (AP), −3.0; medial-lateral (ML), ±4.8; dorsoventral (DV), −7.8, 0.1 μl over 2 min; site 2: AP, −2.8; ML, ±4.8; DV, −7.8, 0.2 μl over 2 min. OFC lesions were as follows: site 1: AP, +4.0; ML, ±0.8; DV, −3.4, 0.2 μl over 3 min; site 2: AP, +3.7; ML, ±2.0; DV, −3.6, 0.3 μl over 2 min; site 3: AP, + 3.2; ML, ±2.6; DV, −4.4, 0.3 μl over 3 min. The AP coordinate was taken from bregma, the ML coordinate from the midline, and the DV coordinate from dura. Animals remained in their home cages for at least 1 week following surgery to allow the animals to recover. During this time, water was available ad libitum and rats were fed ∼20–25 g of chow per day. Animals were initially separated but were housed again in pairs, if possible, following recovery. Eight animals from the lesion groups and three from the sham groups were excluded due to poor recovery.
Experimental design
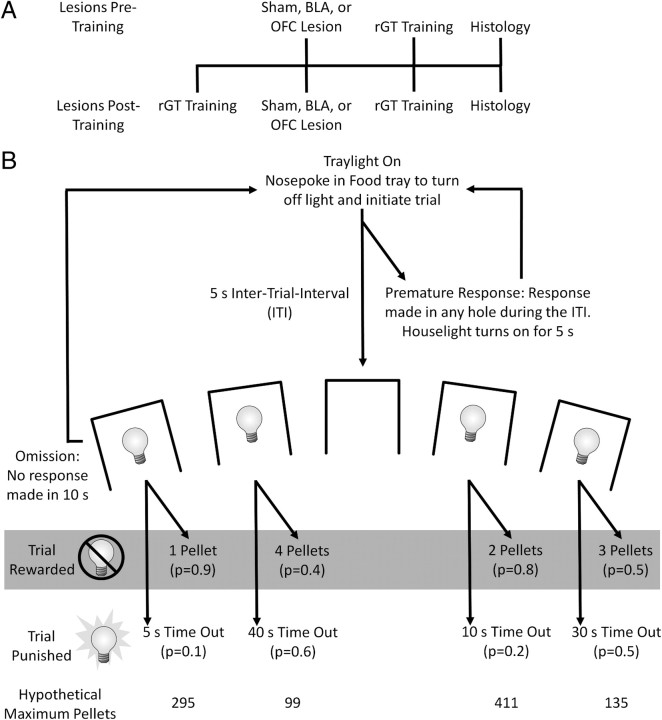
A timeline of the experiments is provided in Figure 1. Animals received BLA or OFC lesion or sham surgery either before or after sufficient training on the rGT. To assess the effects of BLA or OFC lesions on task acquisition, animals that received lesion or sham surgery before rGT training were classified as the acquisition group. Thus, animals in the performance group received surgery following rGT training and were then retested on the rGT.
Figure 1.
Experimental timeline and rGT schematic. A, Timeline indicating when rGT training occurred and when lesion or sham surgery was performed in the two groups. B, Trial structure of the rGT. The p values refer to the probability of a trial resulting in reward or a punishing time-out period. The magnitude of the reward or duration of the time-out period is indicated beside each option. The maximum numbers of pellets that could be obtained if an animal chose a single option exclusively within a single session, assuming each trial lasted 5 s, are listed at the bottom of the diagram, indicating that the two-pellet option, P2, is the best option (schematic modified from Zeeb et al., 2009).
Rat gambling task training and testing
Apparatus.
Detailed descriptions of the testing chambers have been described previously (Carli et al., 1983; Zeeb et al., 2009). Briefly, testing took place in standard five-hole (Med Associates) or nine-hole (Paul Fray) operant chambers. In the nine-hole apparatus, every other hole was blocked so that only five of the holes were accessible. Within the chambers, stimulus response holes were positioned 2 cm above a metal bar floor. A stimulus light was located at the back of each hole. A food tray with a tray light placed therein was located on the opposite wall. Nose-poke responses into the response holes or food tray were detected by a horizontal infrared beam. Sucrose pellets (45 mg; Bioserv) could be delivered to the food tray from an external pellet dispenser. Illumination of the entire chamber could be accomplished by a house light, located at the top of the chamber. All operant chambers were contained within a ventilated and sound-attenuating box. The chambers were controlled by software written in Med PC or BBC BASIC by C.A.W., running on an IBM-compatible or Acorn Archimedes series computer.
Training.
Animals were trained according to previously described methods (Zeeb et al., 2009). Briefly, animals were first habituated to the operant chambers and trained to make nose-poke responses into an illuminated response hole within 10 s following a fixed ratio 1 schedule. Once animals were consistently completing 90–100 trials with ≥80% trials correct and ≤20% trial omitted, they were trained on a forced-choice version of the rGT for seven sessions, where only one of the four possible options was presented on each trial (Zeeb et al., 2009). This ensured all animals had an equal experience with the reward and punishment schedules for all options, thereby preventing the development of any simple biases toward a particular hole.
The rGT.
The design of the rGT has been previously described (Zeeb et al., 2009) and a diagram of the trial structure and reinforcement schedules is provided in Figure 1. In brief, animals were tested once daily in a 30 min session. A trial began when a nose-poke response was made into the illuminated food tray. The tray light was subsequently turned off, initiating a 5 s intertrial interval (ITI). A response at the array during the ITI was classified as a premature response and signaled by illumination of the house light for 5 s, after which the tray light was turned on, allowing the subject to restart the trial. Following the ITI, stimulus lights within holes 1, 2, 4, and 5 were illuminated. If the subject did not respond at the array for 10 s, the trial was scored as an omission and the tray was subsequently illuminated, allowing the subject to start another trial. A nose-poke response in any of these holes turned off the stimulus lights. If the trial was rewarded, the tray light was illuminated and the appropriate number of sucrose pellets immediately delivered into the food tray. Collection of the reward initiated the next trial. If the trial was not rewarded, no food pellets were given and the stimulus light within the chosen hole flashed at 0.5 Hz for the duration of the punishing time-out period. At the end of the punishment period, the tray light was illuminated, allowing the subject to initiate the next trial. Perseverative responses made at the array or food tray following a reward or during the time-out periods were recorded, but not punished.
The reinforcement schedules were designed such that the two-pellet choice (P2) was optimal, in that this option would result in the most reward earned per unit time. Selection from any other option (one-, three-, or four-pellet options) yielded less reward per unit time as a consequence of the probability of winning or losing and the duration of the punishing time-out periods associated with each option (Fig. 1) (Zeeb et al., 2009).
The location of each option (P1–P4) was counterbalanced across all animals, such that half the animals were tested on version A, and half on version B. From left to right, the order of the options presented in version A was P1, P4, P2, P3. In version B, the order of the options presented was P4, P1, P3, P2. Training was considered to be sufficient when a statistically stable pattern of choice and premature responses was observed across at least three sessions (i.e., where p > 0.05 in a repeated-measures ANOVA for either choice × session or session) (Zeeb et al., 2009). The number of postlesion training sessions was determined by stability, as well as by ensuring the animals in both the acquisition and performance groups received a comparable number of training sessions following surgery. As such, animals in the pretraining lesion group (i.e., animals that received lesions before any rGT training) were trained for 30–35 free-choice sessions. Animals in the posttraining group received 30–33 free-choices sessions before surgery, then 33–35 posttraining free-choice sessions.
Data analysis
The main variables analyzed included the percentage choice of each pellet option (number of choices of a particular option/total number of choices made × 100). To analyze the pattern of choice throughout rGT training, the first 20 training sessions were divided into four bouts of 5 d. Choice scores were also assessed, which were calculated as the sum of the two best options (average percentage choice of P1 and P2), the sum of the two worst options (average percentage choice of P3 and P4), and the difference between these two variables. We also analyzed the percentage of premature responses (number of premature responses/total number of trials × 100) and the percentage of omissions (number of omissions/total number of trials × 100) made during each rGT session. All variables expressed as a percentage were subjected to an arcsine transformation before statistical analysis to limit the effect of an artificially imposed ceiling (McDonald, 2009). The total number of trials completed, number of perseverative responses made following both reward and punishment, and latency to respond and collect reward were also analyzed.
Statistical analysis was conducted using SYSTAT for Windows (version number 12.00.08; SSI). All data were analyzed using repeated-measures ANOVA with choice (four levels, P1–P4) and/or session as within-subjects factors, and group (lesion vs sham) as a between-subjects factor. If the outcome of the main ANOVA yielded significant effects at the p < 0.05 level, further post hoc analyses were performed. Values for choice scores and individual sessions or choice options were analyzed using paired sample t tests when comparing data within a single group, or a two-sample t test when comparing data between groups. The p value was determined from either the pooled or separate variance, depending on the outcome of a hypothesis test of variance.
In keeping with previous results (Zeeb et al., 2009), statistical analysis of the percentage choice of each option indicated that there was a significant effect of choice in all groups throughout the entire experiment (for example, choice: F(3,24) = 13.000, p < 0.001). Analysis with version as a between-subjects factor revealed that the order the options were presented did not significantly affect the ability of the animals to perform the task [for example, choice: version: F(1,8) = 0.356, not significant (NS); choice × version: F(3,24) = 1.340, NS; session × choice × version: F(6,48) = 0.423, NS).
In both the acquisition and performance experiments, animals that received either BLA or OFC sham lesions were combined to form a single group. In the acquisition experiment, all rats were run as a single study. Furthermore, when comparing the animals receiving sham lesions to either the BLA or OFC, the postoperative average of either the choice or premature responding data from these sham groups did not differ from each other (choice: choice × sham group: F(3,24) = 0.091, NS; prematures: sham group: F(1,8) = 0.097, NS). As such, data from these rats were pooled to represent a single sham-lesion group. Although some of the rats in the performance group were different strains and were tested on separate occasions, data from animals that received either BLA or OFC sham surgery in these experiments were also not significantly different from each other following surgery (choice: choice × sham group: F(3,51) = 1.750, NS; prematures: sham group: F(1,17) = 0.388, NS). Therefore, data from these two sham groups were also pooled.
Histology
Following completion of behavioral testing, animals were killed by exposure to carbon dioxide. The brains were removed and postfixed in 4% phosphate-buffered paraformaldehyde for 24 h before being stored in a 30% sucrose solution. Brains were sliced into 40 μm sections throughout the area of interest and stained with cresyl violet. The extent of the lesions were determined and mapped onto standardized sections of the rat brain (Paxinos and Watson, 1998).
Results
Lesion analysis
Representative examples of either a BLA or OFC lesion compared with a sham control are shown in Figure 2. Three animals from the OFC lesion group and six from the BLA lesion group were excluded because the lesions were either incomplete, unilateral, or extended far into the surrounding regions. Due to neuronal damage surrounding the infusion site, one animal from the OFC sham group and three from the BLA sham group were also removed from the study. Thus, the number of rats remaining were 24 rats with BLA lesions (acquisition, n = 12; performance, n = 12), 20 with OFC lesions (acquisition, n = 11; performance n = 9), and 29 sham lesion rats (acquisition, n = 10; performance, n = 19).
Figure 2.
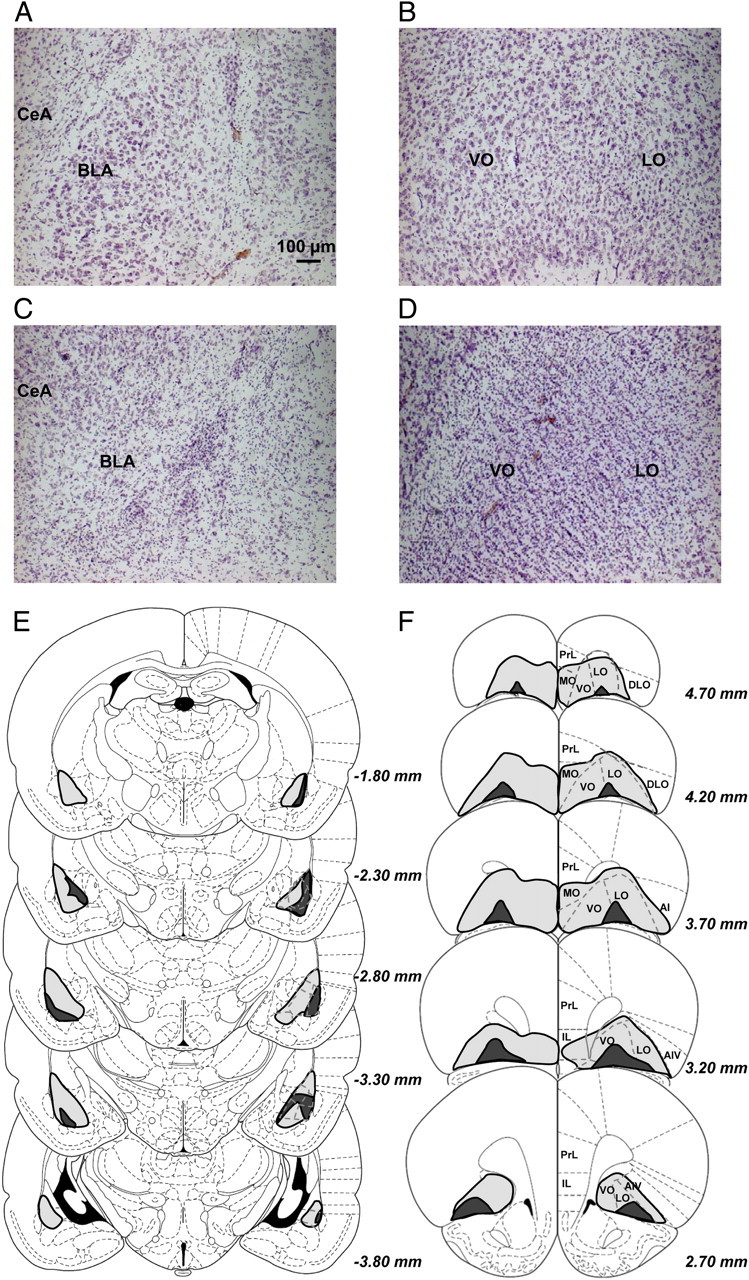
Location and extent of excitotoxic lesions. A–D, Photomicrographs of sham control (A, B) and typical BLA and OFC lesion (C, D) tissue, stained with cresyl violet. E, F, An illustration outlining the boundaries of the largest (gray) and smallest (black) area affected in any one section are shown for both the BLA (E) and OFC (F) lesion groups. Numbers beside each section in E and F correspond to the distance from bregma in millimeters. AI, Agranular insular cortex; AIV, agranular insular cortex ventral part; amygdala; CeA, central amygdala; DLO, dorsolateral prefrontal cortex; IL, infralimbic cortex; LO, lateral orbitofrontal cortex; MO, medial orbitofrontal cortex; PrL, prelimbic cortex; VO, ventral orbitofrontal cortex.
Effects of BLA or OFC lesions on acquisition of the rGT
rGT decision making
Similar to previous results (Zeeb et al., 2009), animals changed their pattern of choice throughout rGT training (sham: day: F(19,152) = 3.549, p = 0.001; day × choice: F(57,456) = 1.952, p < 0.001; BLA: day: F(19,190) = 2.846, p < 0.001; day × choice: F(57,570) = 5.517, p < 0.001; OFC: day: F(19,171) = 7.693, p < 0.001; day × choice: F(57,513) = 4.628, p < 0.001) (Fig. 3). This training effect did not differ between animals tested on version A or B of the rGT (version: all Fs < 1.3, NS; day × version: all Fs < 1.3, NS). However, animals with either BLA or OFC lesions took longer to develop a strong preference for P2, the most optimal option, compared with sham-operated control animals (day × choice × lesion: BLA vs sham: F(57,1140) = 1.595, p = 0.004; OFC vs sham: F(57,1083) = 1.851, p < 0.001). During training, both groups of lesioned rats showed a reduced preference for P2 and an increased choice of P1 compared with sham-operated rats (P2: sham vs BLA, day × lesion: F(19,380) = 2.247, p = 0.002; sham vs OFC, day × lesion: F(19,361) = 2.538, p < 0.001; P1: sham vs BLA, day × lesion: F(19,380) = 1.626, p = 0.05; sham vs OFC, day × lesion: F(19,361) = 1.694, p = 0.04).
Figure 3.
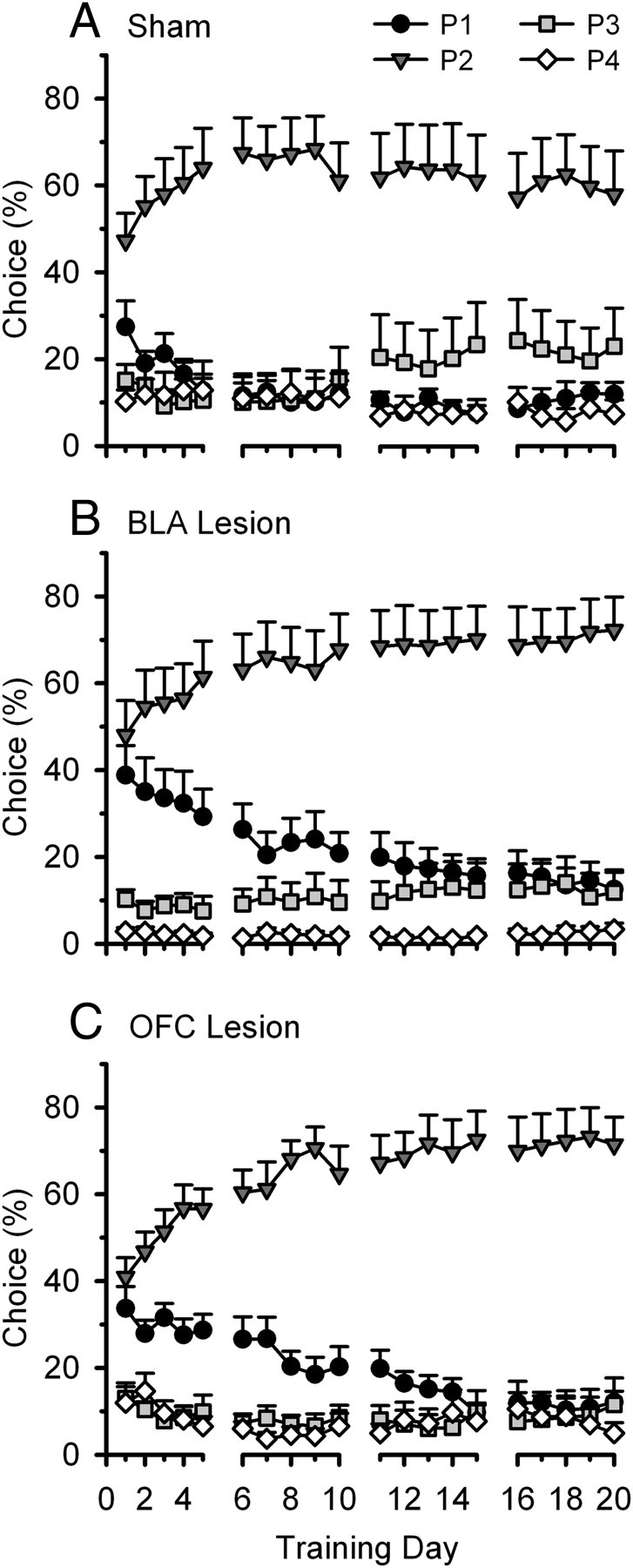
Acquisition of the rGT. A, Animals with sham lesions showed a rapid increase in choice of the two-pellet option, P2, during the first five free-choice training sessions, while subsequently decreasing choice of the less advantageous options. B, C, In contrast, animals with BLA (B) or OFC (C) lesions were slower to learn the rGT, choosing P1 more often and P2 less often during the initial training stages compared with sham-operated control rats. These data indicate that determining the best option appears to be more difficult for both lesion groups during task acquisition.
Rats with BLA lesions also chose P4 slightly less throughout rGT training, although they did not differ in their choice of P3 (P3: lesion: F(1,20) = 0.509, NS; day × lesion: F(19,380) = 1.173, NS; P4: lesion: F(1,20) = 4.994, p = 0.04; day × lesion: F(19,380) = 1.275, NS). In contrast, animals with OFC lesions did not differ from sham lesion rats in their choice of P4, but chose the other disadvantageous option, P3, slightly less often (P3: lesion: F(1,19) = 1.123, NS; day × lesion: F(19,361) = 1.747, p = 0.03; P4: lesion: F(1,19) = 0.183, NS; day × lesion: F(19,361) = 0.949, NS).
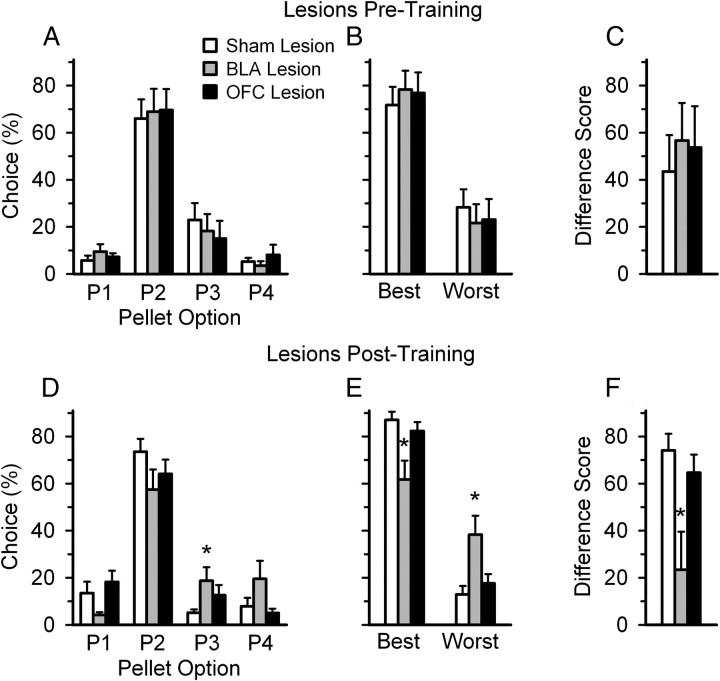
Despite these initial differences, neither lesion resulted in long-term impairments on the rGT. Animals with either BLA or OFC lesions established a similar pattern of decision making compared with sham-operated controls once training was complete (BLA vs sham: lesion: F(1,18) = 1.425, NS; choice × lesion: F(3,54) = 1.621, NS; OFC vs sham: lesion: F(1,17) = 1.372, NS; choice × lesion: F(3,51) = 2.534, NS) (Fig. 4). Furthermore, neither lesion group differed from controls when their combined choice of the advantageous over disadvantageous options and their difference score were compared (BLA vs sham: best: t(20) = 0.735, NS; worst: t(20) = −0.735, NS; difference score: t(20) = 0.586, NS; OFC vs sham: best: t(19) = 0.574, NS; worst: t(19) = −0.574, NS; difference score: t(19) = 0.437, NS) (Fig. 4).
Figure 4.
Choice patterns of rats with BLA or OFC lesions. A–C, Once training had been completed (30–35 postoperative free-choice sessions), rats with pretraining lesions of the BLA chose advantageously, and did not differ from sham-operated control rats in their overall choice (A), discrimination between the good and bad options (B), or choice score (C). D–F, In contrast, rats which received BLA lesions following rGT training chose the disadvantageously (D, E), resulting in a significantly lower choice score (F) compared with sham-operated controls. In contrast, rats with either pretraining or posttraining OFC lesions did not differ from sham-operated rats. Data are expressed as the mean ± SEM. of the last three stable postoperative sessions. Asterisk (*) indicates a significant difference (p < 0.05) determined by a two-sample t test comparing the BLA lesion group to the sham-operated control group.
Other variables
Animals with lesions of the BLA or OFC did not differ from sham-operated controls in the level of premature responding observed during training (BLA vs sham: lesion: F(1,20) = 0.108, NS; day × lesion: F(29,580) = 1.259, NS; OFC vs sham: lesion: F(1,19) = 1.344, NS; day × lesion: F(29,551) = 1.372, NS) (data not shown). Likewise, all groups made similar numbers of premature responses once they had successfully acquired the task (OFC vs sham: lesion: F(1,19) = 0.350, NS) (Table 1). Similarly, lesions of either the BLA or OFC did not significantly affect the latency to choose or collect reward, the number of omissions, or the number of trials initiated (lesion: choice latency: all Fs < 0.239, NS; collection latency: all Fs < 0.442, NS; omissions: all Fs < 3.016, NS; trials: all Fs < 1.251, NS) (Table 1). Although OFC-lesioned rats made more perseverative responses than either sham-operated controls or BLA-lesioned rats, this observation was not significant (lesion: all Fs < 1.443, NS) (Table 1).
Table 1.
Behavioral measurements observed on the rGT following surgery
| Acquisition groups |
Performance groups |
|||||
|---|---|---|---|---|---|---|
| Sham lesion | BLA lesion | OFC lesion | Sham lesion | BLA lesion | OFC lesion | |
| Trials initiated | 99.20 ± 7.49 | 110.11 ± 11.21 | 114.03 ± 10.64 | 123.09 ± 7.63 | 98.58 ± 9.06 | 99.67 ± 5.18 |
| Omissions | 2.33 ± 1.16 | 1.83 ± 0.55 | 0.33 ± 0.16 | 1.33 ± 1.01 | 0.93 ± 0.57 | 1.28 ± 0.83 |
| Choice latency | 1.31 ± 0.18 | 1.17 ± 0.51 | 1.21 ± 0.16 | 1.18 ± 0.18 | 1.08 ± 0.18 | 1.57 ± 0.24 |
| Collection latency | 1.00 ± 0.09 | 0.95 ± 0.07 | 1.09 ± 0.10 | 0.99 ± 0.04 | 0.75 ± 0.06 | 1.44 ± 0.11 |
| Premature responses | 11.91 ± 2.56 | 19.70 ± 3.61 | 13.89 ± 2.17 | 12.29 ± 1.82 | 15.50 ± 3.06 | 20.05 ± 2.93 |
| Perseverative responses | 122.20 ± 19.88 | 123.39 ± 13.69 | 165.52 ± 29.21 | 116.91 ± 16.42 | 188.97 ± 29.09 | 148.15 ± 17.50 |
Data are expressed as the mean ± SEM for the last three postoperative sessions. Omissions and premature responses are represented as a percentage. Latency values are in seconds.
Effects of BLA or OFC lesions on performance of the rGT
rGT decision making
Animals with BLA lesions, but not OFC lesions, chose the disadvantageous options more often than sham-operated rats when lesions were administered following rGT training (Fig. 4). Rats with posttraining BLA lesions chose both the P3 and P4 options more often than sham-operated animals (lesion: F(1,29) = 5.671, p = 0.02; choice × lesion: F(3,87) = 2.882, p = 0.04; session × choice × lesion: F(6,174) = 3.487, p = 0.003; P1: t(29) = −1.635, NS; P2: t(29) = −1.078, NS; P3: t(29) = 2.674, p = 0.01; P4: t(29) = 1.787, p = 0.08). Furthermore, when the animals' choice of the advantageous and disadvantageous options were combined, BLA-lesioned rats chose the best options significantly less and the worst options significantly more compared with sham controls (best: t(29) = −3.193, p = 0.003; worst: t(29) = 3.193, p = 0.003), resulting in a smaller choice score compared with sham-operated controls (t(29) = −2.877, p = 0.01). Together, these results indicate that rats with posttraining lesions of the BLA were significantly impaired in making advantageous decisions on the rGT. In contrast, lesions to the OFC did not alter the animals' ability to choose optimally on the rGT (choice: lesion: F(1,26) = 0.340, NS; choice × lesion: F(3,78) = 1.808, NS; session × choice × lesion: F(6,156) = 1.503, NS; score: best: t(26) = −1.180, NS; worst: t(26) = 1.180, NS; difference score: t(26) = −0.816, NS) (Fig. 4).
Other variables
In contrast to the null effect observed if rats received OFC lesions before task acquisition, lesions of the OFC after the task was acquired tended to increase the number of premature responses made, whereas BLA lesions did not affect this behavior (lesion: BLA vs sham: F(1,29) = 1.005, NS; OFC vs sham: F(1,26) = 2.967, p = 0.09) (Table 1). Although BLA or OFC lesions did not affect the number of trials omitted, these lesions caused animals to initiate fewer trials compared with sham-operated controls (lesion: omissions: all Fs < 2.035, NS; trials completed: BLA vs sham: F(1,29) = 4.180, p = 0.05; OFC vs sham: F(1,26) = 4.004, p = 0.06) (Table 1). A decrease in the number of trials initiated in the BLA lesion group may be due to the observed increase in disadvantageous decision making in this group. Increased choice of the maladaptive options would result in a greater amount of punishment per session, and therefore fewer opportunities to initiate trials. Neither BLA nor OFC lesion animals were slower than sham rats in choosing an option, although rats with either lesion were slower to collect reward (lesion: choice latency: all Fs < 1.530, NS; collection latency: BLA vs sham: F(1,29) = 10.754, p = 0.003; OFC vs sham: F(1,26) = 20.145, p < 0.001) (Table 1). Even though animals in both the BLA and OFC lesion groups made substantially more perseverative responses, this effect reached significance only in the BLA lesion group (lesion: BLA vs sham: F(1,29) = 5.430, p = 0.03; OFC vs sham: F(1,26) = 1.356, NS) (Table 1).
Discussion
These results further delineate the contributions made by the BLA and OFC to decision-making processes using a rodent task conceptually similar to the IGT. Damage to these regions had different effects depending on when the lesion was administered. Lesions of the BLA or OFC made before rGT training significantly slowed learning, but did not result in long-term decision-making impairments. In contrast, animals that received BLA lesions following rGT training persistently favored the disadvantageous options, mimicking the choice pattern of amygdala-damaged patients performing the IGT (Bechara et al., 1999), whereas OFC lesions made posttraining had no such adverse effects. These observations may help to resolve some discrepancies within the human decision-making literature, and confirm that the BLA and OFC have cooperative, yet dissociable, functions in mediating decision-making processes.
Although damage to the OFC impaired task acquisition, lesions induced after the rGT had been learned did not impair choice behavior. As reviewed earlier, poor IGT performance was originally observed in patients with damage to the VMPFC, which encompasses the OFC, in that these subjects preferred decks associated with larger rewards, but also heavier losses (Bechara et al., 1994, 1999). However, patients with comparable brain damage were unimpaired when performing a shuffled version of the IGT, in which gains and losses are distributed randomly throughout each deck (Fellows and Farah, 2005). Eliminating the reversal learning component therefore appeared to resolve the deficit caused by OFC lesions. This fits with previous demonstrations that damage to the OFC results in reversal-learning impairments (Dias et al., 1996; McAlonan and Brown, 2003; Ragozzino, 2007; Schoenbaum et al., 2007; Rudebeck and Murray, 2008; Kazama and Bachevalier, 2009; Robbins and Arnsten, 2009). The rGT resembles this shuffled version, in that rewards or punishments occur randomly (Zeeb et al., 2009). OFC lesions made after the task was learned did not affect choice patterns, such that the data from both the rat and human experiments are concordant.
However, it seems unlikely that the VMPFC/OFC has no role in mediating judgments based on risk and uncertainty. Although very discrete lesions of the OFC do not result in IGT impairments in human subjects (Manes et al., 2002), OFC-damaged patients are impaired on a different decision-making task that likewise taxes judgments involving probabilistic rewards and punishments (Rogers et al., 1999b), and neuroimaging data indicate that the OFC is recruited in such tasks (Rogers et al., 1999a; Cunningham et al., 2009; Lawrence et al., 2009; Hartstra et al., 2010). There is also an inconsistency between human data and the findings presented here, in that the performance of rats with posttraining OFC lesions matches findings from patients who had not learned the IGT before their brain injury. This latter observation may contribute to a parsimonious explanation for the different effects of OFC lesions reported to date, in that the level of learning required to assimilate the task may be a critical factor in determining lesion outcome.
From a theoretical perspective, lesions made before training unmask the behavioral output of a network forced to learn task contingencies without the input of the targeted area. In contrast, lesions made subsequent to task acquisition demonstrate the consequences of disrupting an already-established decision-making network. A direct comparison of the two manipulations therefore indicates whether nodes that are critical for learning are likewise fundamental for execution. In the current study, damaging the BLA or OFC before rGT acquisition had similar, detrimental effects on animals' ability to learn the optimal strategy. The strong resemblance between the effects of both lesions suggest that these impairments arose due to a disruption in the functional connectivity between these two regions, a pathway known to be important in the regulation of goal-directed behavior (Baxter et al., 2000). Future experiments will aim to test this hypothesis. However, following sufficient training, animals with either lesion were able to perform the rGT optimally. This pattern of data implies that cross talk between these regions is especially important while subjects are learning the response contingencies, but that other areas are ultimately capable of compensating for their loss.
It has been suggested that the OFC encodes outcome expectancies, and previous work suggests that input from the BLA is important in allowing the OFC to maintain accurate and up-to-date representations of the subjective value of difference response options (Schoenbaum and Esber, 2010). Likewise, input from the OFC to the BLA is needed in order for this region to optimally identify stimuli that are predictive of reward (Stalnaker et al., 2007). Such an interaction would be particularly important when subjects are learning the IGT/rGT. If the rules of a task are easily assimilated, such that this learning mechanism is not overly taxed, then the effects of OFC damage should theoretically be minimal. Indeed, some reports indicate that OFC lesions only impair reversal learning during early reversals when cognitive demands are relatively high and the rules of the task are still being acquired (Dias et al., 1996; Schoenbaum et al., 2002; Boulougouris et al., 2007; Boulougouris and Robbins, 2009). In the shuffled version of the IGT, which is not impaired in OFC-damaged patients, subjects appear to adopt the correct strategy almost immediately, suggesting that learning occurs rapidly and the task contingencies are more transparent (Fellows and Farah, 2005). In contrast, there is a marked within-session learning curve in the original IGT because the decks are stacked, making it more difficult to decipher the correct strategy. As such, OFC-lesioned subjects fair poorly (Bechara et al., 1994, 1999). Although VMPFC damage has been reported to impair performance of the Cambridge gamble task, in which learning and memory requirements are minimal, surrounding cortical areas (such as Brodmann's areas 10 and 32) were also damaged in this patient group (Clark et al., 2008), whereas patients with discrete lesions of the OFC were unimpaired on this task (Manes et al., 2002).
In the current study, both BLA- and OFC-lesioned rats were slower to acquire the optimal strategy on the rGT due to a higher preference for the one-pellet option (P1). This option is associated with the most frequent rewards and the shortest punishing time-outs, and may therefore be the most tempting alternative to P2 even though less reward is obtained in the long run (Zeeb et al., 2009). Although this strategy could not be described as risk-seeking in the way that preference for the disadvantageous options appears to be on the IGT, it may nonetheless reflect a difficulty in overcoming an initial preference. Hence, without a functional connection between the BLA and OFC, both rats and humans find it harder to differentiate between options that appear superficially compelling. It is therefore possible that the increased risky choice observed in the original, unshuffled IGT following both OFC and amygdala damage actually results, at least in part, from a learning impairment rather than from genuinely risk-seeking behavior.
In contrast, rats that received BLA lesions following successful acquisition of the rGT persistently showed a maladaptive choice pattern, favoring the more costly, larger reward options. This may be the first evidence of true risk-seeking behavior following amygdala damage that is independent of learning. These results are also unlikely to reflect an inability to adapt to changing task contingencies, such as those present in probability-discounting tasks (Ghods-Sharifi et al., 2009). It could be argued that this lesion-induced decline in rGT performance reflects a memory deficit, in that lesioned animals were unable to recall the correct strategy following surgery. This appears unlikely, as preference for the disadvantageous options in BLA-lesioned rats became more pronounced with repeated postoperative training and ultimately remained stable until the end of the experiment (33–35 postsurgery training sessions). Persistent deficits in fear-conditioning that do not recover following extensive training have likewise been observed when BLA lesions are performed after, but not before, task acquisition (Maren et al., 1996, 1999). The idea that BLA damage may induce reckless or risk-seeking behavior is consistent with clinical observations that such patients are generally unable to live independently due to their tendency to pursue actions that endanger themselves and others (Lee et al., 1988a,b, 1995). The IGT impairments observed in amygdala-lesioned patients were attributed to a decreased sensitivity to rewarding and punishing outcomes, as these patients failed to generate skin conductance responses in anticipation of making a risky choice, or following rewarding or punishing outcomes (Bechara et al., 1999). In the current study, BLA-lesioned rats were still capable of discriminating between the different options, suggesting they are not indifferent to the nature of the response outcome. However, the BLA has been shown to play an important role in mediating the behavioral influence of stimuli conditioned to both positive and negative events (Killcross et al., 1997, Hatfield et al., 1996). An impairment in using such cues could theoretically increase preference for the disadvantageous options due to a weakened association between the response option and the expectation of loss, but this remains to be empirically determined.
In summary, the application of selective lesion techniques has enabled us to parse out the relative contributions of the BLA and OFC in a rodent model of risk-based, real-world decision-making. The BLA appears to be highly important in maintaining an optimal decision-making strategy once it has been acquired, whereas both the BLA and OFC are involved in effectively learning the rules associated with multiple options, potentially through functional and reciprocal connectivity. Improving our knowledge of the neural circuitry controlling this form of decision-making may contribute to a clearer understanding of the psychiatric disorders in which such processes are compromised.
Footnotes
This work was supported by an operating grant awarded to C.A.W. from the Canadian Institutes for Health Research (CIHR). C.A.W. also receives salary support through the Michael Smith Foundation for Health Research and the CIHR New Investigator Award program. We thank Dr. Trevor Robbins and the Behavioural and Clinical Neuroscience Institute at the University of Cambridge for their invaluable support for the initial phase of this experiment. We also thank Hayden Rubensohn for superb technical assistance on this project.
C.A.W. has previously consulted for Theravance on an unrelated matter. The authors have no other conflicts of interest or financial disclosures to make.
References
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Pre-surgical training ameliorates orbitofrontal-mediated impairments in spatial reversal learning. Behav Brain Res. 2009;197:469–475. doi: 10.1016/j.bbr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Kesek A, Mowrer SM. Distinct orbitofrontal regions encode stimulus and choice valuation. J Cogn Neurosci. 2009;21:1956–1966. doi: 10.1162/jocn.2008.21148. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Fellows LK. The role of orbitofrontal cortex in decision making: a component process account. Ann N Y Acad Sci. 2007;1121:421–430. doi: 10.1196/annals.1401.023. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Nijs I, Muris P. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Res. 2008;158:155–163. doi: 10.1016/j.psychres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29:5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstra E, Oldenburg JF, Van Leijenhorst L, Rombouts SA, Crone EA. Brain regions involved in the learning and application of reward rules in a two-deck gambling task. Neuropsychologia. 2010;48:1438–1446. doi: 10.1016/j.neuropsychologia.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Kazama A, Bachevalier J. Selective aspiration or neurotoxic lesions of orbital frontal areas 11 and 13 spared monkeys' performance on the object discrimination reversal task. J Neurosci. 2009;29:2794–2804. doi: 10.1523/JNEUROSCI.4655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O'Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa gambling task. Cereb Cortex. 2009;19:1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Lee GP, Arena JG, Meador KJ, Smith JR, Loring DW, Flanigin HF. Changes in autonomic responsiveness following bilateral amygdalotomy in humans. Neuropsychiatry Neuropsychol Behav Neurol. 1988a;1:119–129. [Google Scholar]
- Lee GP, Meador KJ, Smith JR, Loring DW, Flanigin HF. Preserved crossmodal association following bilateral amygdalotomy in man. Int J Neurosci. 1988b;40:47–55. doi: 10.3109/00207458808985727. [DOI] [PubMed] [Google Scholar]
- Lee GP, Reed MF, Meador KJ, Smith JR, Loring DW. Is the amygdala crucial for cross-modal association in humans? Neuropsychologia. 1995;9:236–245. [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McDonald J. Handbook of biological statistics. Ed 2. Baltimore: Sparky House Publishing; 2009. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edition. Sydney: Academic; 1998. [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999a;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999b;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Esber GR. How do you (estimate you will) like them apples? Integration as a defining trait of orbitofrontal function. Curr Opin Neurobiol. 2010;20:205–211. doi: 10.1016/j.conb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann N Y Acad Sci. 2007;1121:320–335. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Starcke K, Tuschen-Caffier B, Markowitsch HJ, Brand M. Dissociation of decisions in ambiguous and risky situations in obsessive-compulsive disorder. Psychiatry Res. 2010;175:114–120. doi: 10.1016/j.psychres.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]