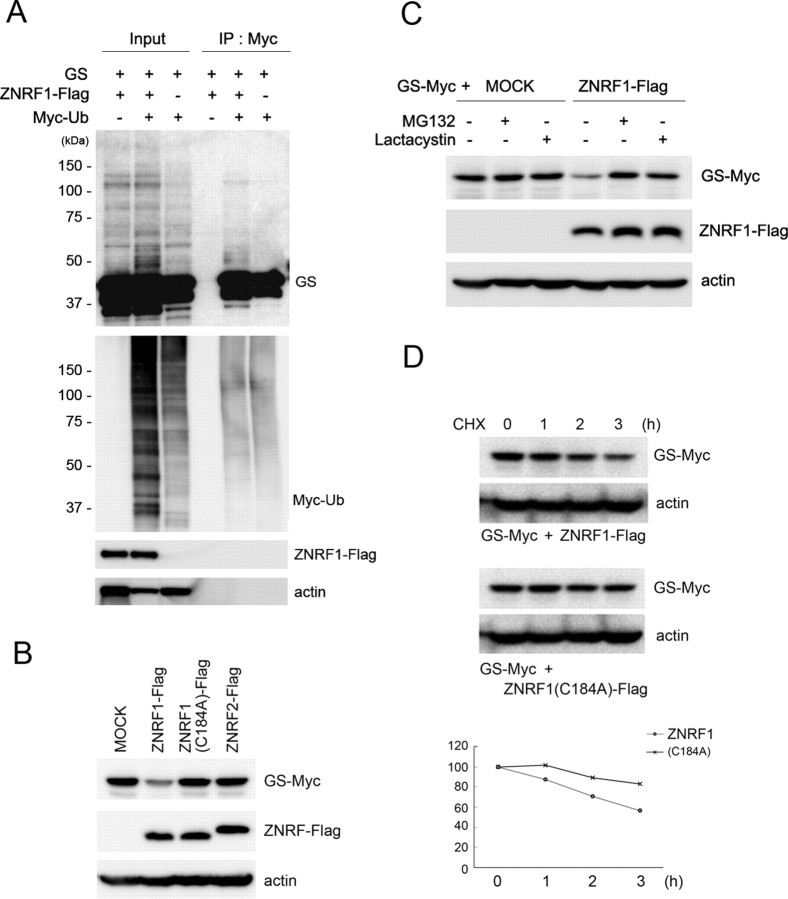
Figure 2.
GS is a substrate for ZNRF1-dependent ubiquitination and proteasomal degradation. A, HEK293 cells were transfected with expression plasmids for GS, ZNRF1-Flag, and/or Myc-tagged ubiquitin, and treated with MG132 (20 μm) for 6 h. Cell lysates were subject to immunoprecipitation with anti-Myc, and ubiquitinated GS in the resultant immunoprecipitates was analyzed by immunoblot using an anti-GS antibody. Note that ubiquitinated GS was detected as a smear in the high-molecular-weight range as indicated. B–D, Neuro-2a cells were transfected with expression plasmids for GS-Myc together with either ZNRF1-Flag, ZNRF1(C184A)-Flag, ZNRF2-Flag, or an empty vector (MOCK), and the cell lysates were subjected to immunoblot analysis to detect GS-Myc expression. Actin served as the loading control. Note that GS immunoreactivity became significantly lower only when wild-type ZNRF1 was present (B). When the cells were treated with 10 μm MG132 or 10 μm lactacystin after transfection for 24 h before lysis, ZNRF1-dependent decrease in GS was inhibited (C). In D, ZNRF1-dependent degradation of GS increased its turnover rate. Neuro-2a cells were transfected with GS-Myc plasmid together with wild-type ZNRF1-Flag or ZNRF1 (C184A)-Flag plasmid. Cell lysates were generated at indicated hours after the addition of cycloheximide and subject to immunoblot analysis to detect GS. Actin served as the loading control. Representative immunoblot and quantitative analysis of GS expression normalized to GS levels at 0 h are shown.