Figure 4.
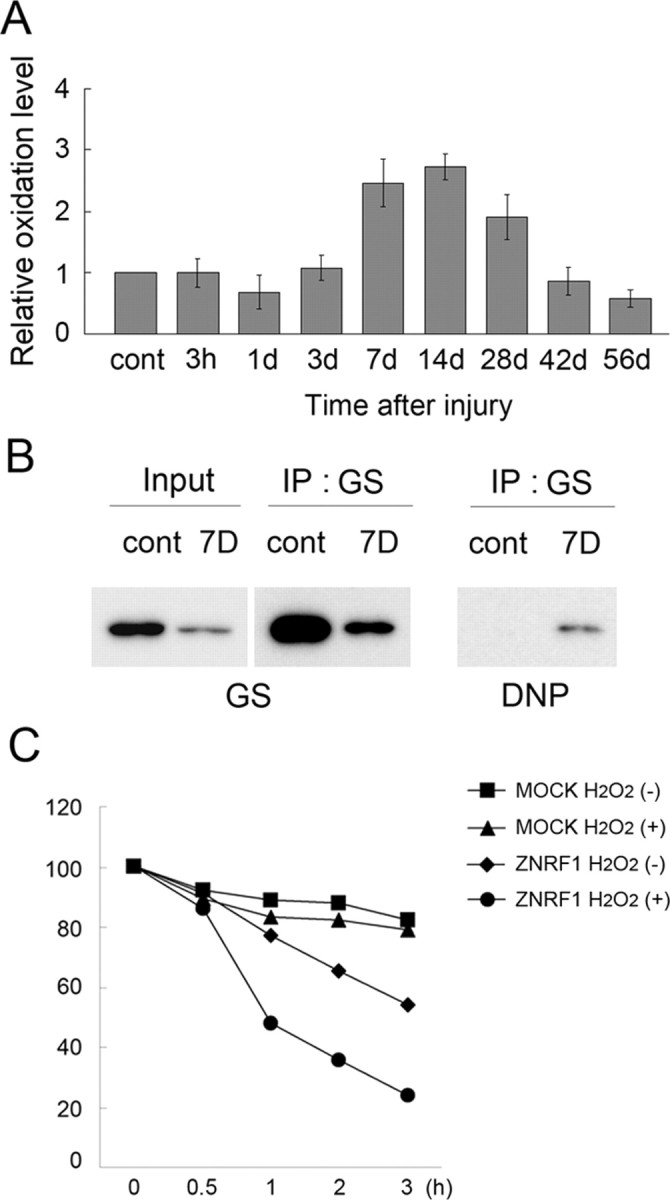
Increased oxidative stress in Schwann cells after nerve injury induces GS oxidization and degradation. Adult mouse sciatic nerves were crushed, and the distal nerve stump was collected at the indicated time after crush. A, Tissue lysates were analyzed for oxidized protein using OxyBlot-protein oxidation detection kit, in which carbonyl groups in the oxidized protein side chains were derivatized to 2,4-dinitrophenylhydrazone (DNP) by reacting with 2,4-dinitrophenylhydrazine (DNPH). The DNP-derivatized protein samples were subjected to immunoblot analysis using an anti-DNP antibody. Carbonylated protein expression level at each indicated time point after injury was normalized to actin and shown relative to the expression level in uninjured control nerves. B, Lysates of nerve tissues (uninjured control and injured nerve 7 d after crush) were subject to immunoprecipitation to detect DNP-derivatized GS. C, ZNRF1-dependent GS degradation in Schwann cells is induced by oxidative stress. Neuro-2a cells transfected with expression plasmids for GS-Myc together with ZNRF1-Flag or an empty vector (MOCK) were treated with H2O2 for 4 h. Both H2O2-treated and untreated cell lysates were collected at indicated hours after the addition of cycloheximide and subjected to immunoblot analysis to detect GS. Quantitative analysis of GS expression normalized to expression levels at 0 h is shown. Note that GS decrease in the presence of ZNRF1 is significantly accelerated by H2O2, suggesting that oxidized GS is preferentially subject to ZNRF1-dependent proteasomal degradation.
