The best non-invasive method to examine the brain is through a detailed neurologic assessment. However, several clinical situations preclude patient participation, which compels providers to rely on alternative means of monitoring neurologic wellbeing.
A major determinant of neurologic outcomes is cerebral hemodynamics, as hypoperfusion and hyperperfusion may result in ischemia and edema, respectively. In health, cerebral autoregulation maintains constant cerebral blood flow (CBF) despite perfusion pressure changes. Lassen’s classic autoregulatory plateau of 50-150 mmHg still informs empiric blood pressure targets used by clinicians in perioperative and critical care settings. There is growing recognition, however, that there is variability between individuals in the contours and limits of the autoregulatory curve and that numerous pathologic states can impair the brain’s autoregulatory capacity (Figure 1).1 Consequently, some patients maintained at what is presumed to be an acceptable blood pressure may be at risk for cerebral hypo/hyperperfusion. This has spurred the desire for tools capable of monitoring cerebral autoregulation in real time as a means of updating our one-size-fits-all approach.
Figure 1.
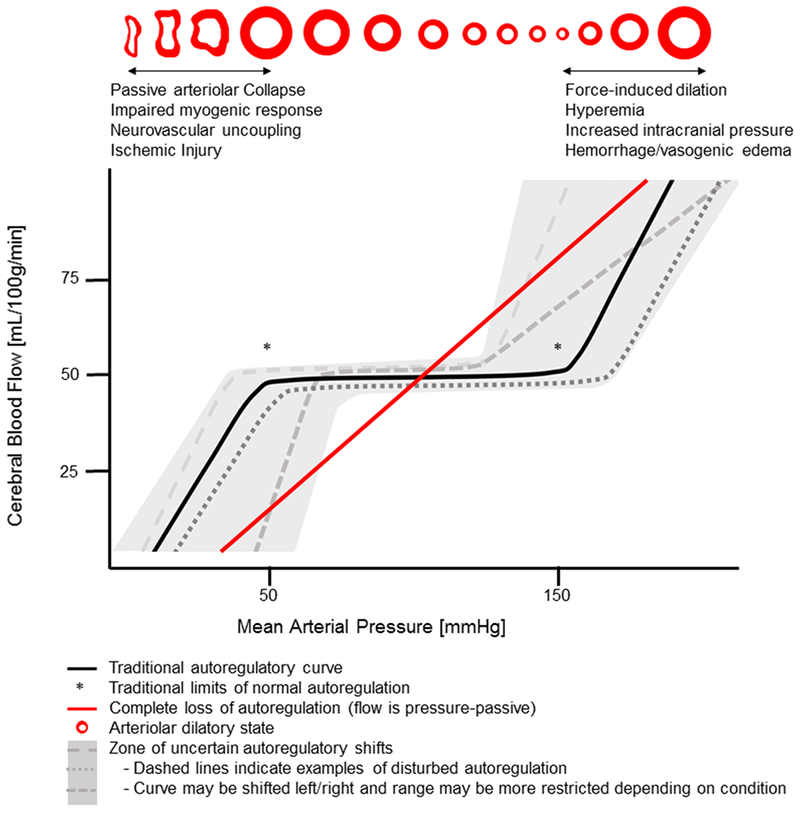
Autoregulation of cerebral blood flow
Cerebral autoregulation assessment requires measurement of CBF or intracranial pressure, for which highly invasive monitors or cumbersome imaging equipment is needed. Thus, clinicians rely on surrogate measures. Transcranial Doppler can be used, but acoustic windows are challenging to obtain and the technique requires frequent probe adjustment. Near-infrared spectroscopy (NIRS) is an appealing choice because it is noninvasive, does not require advanced expertise and can be measured continuously for prolonged periods of time. Cerebral NIRS makes use of the differential light absorption spectra of two abundant biologic chromophores, oxyhemoglobin and deoxyhemoglobin, to determine their relative concentrations. Because plethysmography is not used, cerebral NIRS signals reflect the mixed arterial and venous oxygenation of the frontal lobe tissue in the path of the emitted light. Fluctuations in tissue oxygenation can be attributed to changes in CBF when other determinants (arterial oxygen saturation, hemoglobin, tissue diffusivity of oxygen, and cerebral metabolic rate of oxygen) are presumed to be held constant.2 Depending on the manufacturer and the particular methodology employed, NIRS devices generate various measures including regional cerebral oxygen saturation (rScO2), tissue oxygenation index (TOI), fractional tissue oxygen extraction (cFTOE), relative total tissue hemoglobin concentration (rTHb), and cerebral blood flow index (rCBFi). The latter is measured with diffuse correlation spectroscopy, a newer technology that detects blood flow by quantifying temporal fluctuations of near-infrared light propagating diffusely through brain tissue and being scattered by red blood cells in the microvasculature. NIRS can also monitor neuronal oxygen metabolism by measuring changes in the oxidation state of cytochrome c oxidase (oxCCO), another near-infrared attenuating biologic chromophore.
Cerebral autoregulation analyses involve quantifying the relationship between perfusion pressure and cerebral blood flow or volume using various mathematical models. In general, a high correlation between NIRS-derived measures of flow (or volume) and perfusion pressure (high or positive index of autoregulation) indicates disturbed autoregulation. Many groups have used a time-domain approach, either with a simple, moving correlation coefficient between pressure and flow (or volume) (COx for rScO2, TOx for TOI, HVx for rTHb, BFAx for rCBFi) or with linear regression.3–6 Alternatively, frequency domain analysis, which involves examining the relationship between pressure and flow in specific frequency bands, can be used.7 A potential advantage of this method is that it allows for the possibility of various autoregulatory responses occurring over different time courses. However, when relying on spontaneous, nonstationary fluctuations in pressure, this approach may not be feasible. More recently, continuous wavelet transform methodology has been employed.8 This technique considers the nonstationary aspect of autoregulation by combining both temporal and frequency signal features.
The number of different patient groups for which NIRS-based autoregulation monitoring has been validated against other measures, such as xenon clearance or transcranial Doppler, continues to grow and includes: preterm infants, critically ill adults with and without primary brain injury, patients at risk for cerebral hypoperfusion, such as those undergoing neurovascular, cardiovascular, or beach-chair position shoulder surgery. In many of these patient populations, investigators have shown that dysautoregulation or cerebral hypo/hyperperfusion identified by autoregulation monitoring is associated with significant morbidity and mortality. Notably, recent studies have demonstrated that monitoring NIRS-derived autoregulation indices may be a more reliable indicator of cerebral physiologic health and neurologic outcomes than solely monitoring changes in rScO2 or TOI.9 Some studies have further suggested that autoregulation-guided perfusion pressure management, referred to as “optimal blood pressure”, is feasible and may improve outcomes.10
A major obstacle that has limited clinical implementation is a lack of standardization. The variety of commercially-available monitors measure different parameters and employ a number of different proprietary algorithms. Preprocessing techniques such as signal acquisition frequency, data filtering, and artifact removal are also far from uniform among investigators. Additionally, different statistical and graphical techniques are available to generate autoregulation curves. Even after curve creation, the most appropriate index-specific cut-offs and methods for identifying curve features (upper and lower limits or optimal blood pressure) have yet to be determined. Furthermore, the best way to measure the exposure is unclear. Some investigators report the average index of autoregulation, others measure the time blood pressure strays beyond the limits of autoregulation, while some examine the product of time and magnitude of blood pressure outside the limits of autoregulation.
Though we are beginning to understand the pathophysiological mechanisms underlying observed instances of impaired autoregulation, our understanding is far from complete. Elevated autoregulation index values may sometimes be the result of perfusion pressure outside the limits of autoregulation, perhaps from a shifted or narrowed plateau (Figure 1). In some situations, an individual’s capacity for autoregulation may be completely abolished, leading to high autoregulation index values at all blood pressures, or complete dysautoregulation. These autoregulatory disturbances are likely dynamic and may occur in individual patients at different times during the course of illness or with differing anesthetic depth or agents. Continuous and standardized autoregulation monitoring in large and diverse patient populations is necessary to further explore the epidemiology and pathophysiology of impaired autoregulation. Detecting these potentially time-varying autoregulation disturbances is essential to: 1) mitigating primary and secondary brain injury; 2) relating autoregulatory function with clinical outcomes, and; 3) individualizing therapy to optimize cerebral hemodynamics. Furthermore, to improve outcomes, individualized therapeutic targets require real-time assessments and clear methods for defining and detecting optimal blood pressures.
The value added to standalone NIRS monitoring by coupling it with blood pressure to analyze autoregulation remains to be fully demonstrated. To realize the promise of bedside autoregulation monitoring, some of the challenges mentioned above must be overcome. Given the devastating nature of neurologic injury, clinicians should be eager to investigate monitors that might provide actionable information. In the near future, we expect the tide will turn in favor of autoregulation monitoring over standalone NIRS use, as more and more comprehensive, well-designed studies will demonstrate the efficacy of NIRS-based autoregulation monitoring to improve neurologic outcomes.
Acknowledgments
Brian Bush, MD, MHS is supported by grant 5T32GM075774-13 from the National Institutes of Health/National Institute of General Medical Sciences Ruth L. Kirschstein National Research Service Award (T32). Kevin Sam, PhD is supported by the Canadian Institutes of Health Research Postdoctoral Fellowship (MFE-158191) and the Boerger Research Fund for Alzheimer’s Disease and Neurocognitive Disorders from the Foundation of the American Society of Neuroradiology (FASNR).
Footnotes
Competing Interests: The authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Drummond JC. Blood Pressure and the Brain: How Low Can You Go? Anesth Analg. 2019;128(4):759–771. doi: 10.1213/ANE.0000000000004034 [DOI] [PubMed] [Google Scholar]
- 2.Tyszczuk L, Meek J, Elwell C, Wyatt JS. Cerebral Blood Flow Is Independent of Mean Arterial Blood Pressure in Preterm Infants Undergoing Intensive Care. Pediatrics. 1998;102(2):337–341. doi: 10.1542/peds.102.2.337 [DOI] [PubMed] [Google Scholar]
- 3.Brady KM, Lee JK, Kibler KK, et al. Continuous Time-Domain Analysis of Cerebrovascular Autoregulation Using Near-Infrared Spectroscopy. Stroke. 2007;38(10):2818–2825. doi: 10.1161/STROKEAHA.107.485706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-Infrared Spectroscopy can Monitor Dynamic Cerebral Autoregulation in Adults. Neurocrit Care. 2008;10(1):122–128. doi: 10.1007/s12028-008-9140-5 [DOI] [PubMed] [Google Scholar]
- 5.Lee JK, Kibler KK, Benni PB, et al. Cerebrovascular Reactivity Measured by Near-Infrared Spectroscopy. Stroke. 2009;40(5):1820–1826. doi: 10.1161/STROKEAHA.108.536094 [DOI] [PubMed] [Google Scholar]
- 6.Selb J, Wu K- C, Sutin J, et al. Prolonged monitoring of cerebral blood flow and autoregulation with diffuse correlation spectroscopy in neurocritical care patients. Neurophotonics. 2018;5(4):045005. doi: 10.1117/1.NPh.5.4.045005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caicedo A, Alderliesten T, Naulaers G, Lemmers P, van Bel F, Van Huffel S. A New Framework for the Assessment of Cerebral Hemodynamics Regulation in Neonates Using NIRS. Adv Exp Med Biol. 2016;876:501–509. doi: 10.1007/978-1-4939-3023-4_63 [DOI] [PubMed] [Google Scholar]
- 8.Tian F, Tarumi T, Liu H, Zhang R, Chalak L. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. Neuroimage Clin. 2016;11:124–132. doi: 10.1016/j.nicl.2016.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis C, Parulkar SD, Bebawy J, Sherwani S, Hogue CW. Cerebral Neuromonitoring During Cardiac Surgery: A Critical Appraisal With an Emphasis on Near-Infrared Spectroscopy. J Cardiothorac Vasc Anesth. 2018;32(5):2313–2322. doi: 10.1053/j.jvca.2018.03.032 [DOI] [PubMed] [Google Scholar]
- 10.Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, Healy RJ, Ziai W, Mirski MA. Cerebral Autoregulation-oriented Therapy at the Bedside: A Comprehensive Review. Anesthesiology. 2017;126(6):1187–1199. doi: 10.1097/ALN.0000000000001625 [DOI] [PubMed] [Google Scholar]


