Abstract
Increasing evidence suggests that 5-HT1A receptor (5-HT1AR) is implicated in anxiety disorders. However, the mechanism underlying the role of 5-HT1AR in these diseases remains unknown. Here, we show that 5-HT1AR-selective agonist 8-OH-DPAT and selective serotonin reuptake inhibitor (SSRI) fluoxetine downregulated hippocampal neuronal nitric oxide synthase (nNOS) expression, whereas 5-HT1AR-selective antagonist NAN-190 upregulated hippocampal nNOS expression. By assessing anxiety-related behaviors using the novelty suppressed feeding, open-field, and elevated plus maze tests, we show that mice lacking nNOS gene [knock-out (KO)] or treated with nNOS-selective inhibitor 7-nitroindazole (7-NI; i.p., 30 mg/kg/d for 28 d; or intrahippocampal microinjection, 16.31 μg/1.0 μl) displayed an anxiolytic-like phenotype, implicating nNOS in anxiety. We also show that, in wild-type (WT) mice, administrations of 8-OH-DPAT (i.p., 0.1 mg/kg/d) or fluoxetine (i.p., 10 mg/kg/d) for 28 d caused anxiolytic-like effects, whereas NAN-190 (i.p., 0.3 mg/kg/d for 28 d) caused anxiogenic-like effects. In KO mice, however, these drugs were ineffective. Moreover, intrahippocampal infusion of 8-OH-DPAT (45.963 μg/100 μl) using 14 d osmotic minipump produced anxiolytic effects. Intrahippocampal microinjection of 7-NI (16.31 μg/1.0 μl) abolished the anxiogenic-like effects of intrahippocampal NAN-190 (4.74 μg/1.0 μl). Additionally, NAN-190 decreased and 8-OH-DPAT increased phosphorylated cAMP response element-binding protein (CREB) levels in WT mice but not in KO mice. Blockade of hippocampal CREB phosphorylation by microinjection of H89 (5.19 μg/1.0 μl), a PKA (protein kinase A) inhibitor, abolished the anxiolytic-like effects of 7-NI (i.p., 30 mg/kg/d for 21 d). These findings indicate that both hippocampal nNOS and CREB activity mediate the anxiolytic effects of 5-HT1AR agonists and SSRIs.
Introduction
Epidemiological studies have indicated that as many as 29% of people will, at some point in their lives, suffer from anxiety disorders (Hawgood and De Leo, 2008). There is increasing clinical evidence that anxiety disorders are associated with suicidal ideation, suicide attempts, and suicide (Placidi et al., 2000; Khan et al., 2002; Sareen et al., 2005). The serotonergic [5-hydroxytryptamine (5-HT)] system is essential for regulating affects and moods and has long been implicated in the pathogenesis of anxiety disorders. The activity of serotonergic pathways is critically regulated by the plasma membrane serotonin transporter, which removes serotonin released into the synaptic cleft (Torres et al., 2003), and serotonin receptors. To date, over 15 different serotonin receptors have been identified. Among the various 5-HT receptor subtypes, 5-HT1A receptor (5-HT1AR) has been predominantly implicated in the modulation of mood and anxiety-related behaviors (Sargent et al., 2000; Gross et al., 2002). Anxiety and depression are often associated with downregulation of the 5-HT1AR in the hippocampus and in the temporal lobe (Gross et al., 2000) and 5-HT1AR agonists have been used as anxiolytic drugs. Mice that genetically lack 5-HT1AR exhibit anxiety-like behaviors (Gingrich and Hen, 2001; Olivier et al., 2001). Expression of 5-HT1AR in the hippocampus and cortex is sufficient to rescue the behavioral phenotype of the 5-HT1AR knock-out (KO) mice (Gross et al., 2002). Moreover, activation of the 5-HT1AR is a critical component in the mechanism of action of selective serotonin reuptake inhibitors (SSRIs) (Santarelli et al., 2003). However, the mechanism underlying the role of 5-HT1AR in anxiety-related behaviors remains unknown.
Neuronal nitric oxide synthase (nNOS)-derived nitric oxide (NO), a free radical with signaling functions in the CNS, may be an important downstream signaling molecule of 5-HT1AR in regulating anxiety-related behaviors. nNOS is enriched throughout the limbic system (Bredt et al., 1991; Dawson et al., 1991), an area important in emotional behaviors. Our previous study showed that nNOS-derived NO contributes to chronic stress-induced depression (Zhou et al., 2007). Recently, several studies suggest that nNOS may be related to anxiety-related behaviors (Miguel and Nunes-de-Souza, 2008; Workman et al., 2008). More importantly, nNOS plays a role in normal brain 5-HT function, especially in the 5-HT1A and/or 5-HT1B postsynaptic receptor function, and has significant implications for the psychiatric disorders characterized by aggressiveness and impulsivity (Chiavegatto et al., 2001). Here we investigated the relationship of 5-HT1AR and nNOS in anxiety-related behaviors and reported that hippocampal nNOS and cAMP response element-binding protein (CREB) activity mediates the anxiolytic effects of 5-HT1AR agonists and SSRIs.
Materials and Methods
Animals.
Young adult (6- to 7-week-old) male homozygous nNOS-deficient mice (KO mice; B6, 129-NOS1tm1plh, stock number: 002633) and their wild-type (WT) littermates of similar genetic background (B6129SF2) (both from Jackson Laboratories; maintained at Model Animal Research Center of Nanjing University, Nanjing, China), and young adult (6–7 weeks) and newborn [postnatal day (P)0–P1] C57/BL/6 mice (from Model Animal Research Center of Nanjing University, Nanjing, China) were used in this study. The mice were maintained at a controlled temperature (20 ± 2°C) and group housed (12 h light/dark cycle) with access to food and water ad libitum. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. Every effort was made to minimize the number of animals used and their suffering.
Drugs and systemic injections.
Fluoxetine (10 mg/kg/d), 7-nitroindazole (7-NI; 30 mg/kg/d), l-arginine (750 mg/kg/d), d-arginine (750 mg/kg/d), 8-OH-DPAT (0.1 mg/kg/d), NAN-190 (0.3 mg/kg/d), imipramine (20 mg/kg/d), or desipramine (20 mg/kg/d) was intraperitoneally injected. All these drugs were purchased from Sigma-Aldrich.
Intrahippocampal infusions and microinjections.
Adult mice were anesthetized with 0.07 ml of a mixture of ketamine (90.9 mg/ml) and xylazine (9.1 mg/ml) and placed in a stereotaxic apparatus. For the osmotic pump infusions, a 14 d Alzet osmotic minipump containing 8-OH-DPAT (45.963 μg/100 μl) was placed subcutaneously in the back of the animals and a brain infusion cannula connected to the pump was positioned at the following coordinates: 2.3 mm posterior to bregma, 1.3 mm lateral to the midline, and 2.0 mm below dura (Munoz et al., 2005). The infusion rate was 0.25 μl/h. We anesthetized mice with a mixture of ketamine (90.9 mg/ml) and xylazine (9.1 mg/ml) and removed the osmotic pump after infusing 8-OH-DPAT solution for 14 d. For the microinjections, the designated drug solution in 1.0 μl of volume was injected into the hippocampus (0.2 μl/min) at coordinates as above.
Novelty suppressed feeding procedure.
The novelty suppressed feeding (NSF) procedure is a conflict test that elicits competing motivations: the drive to eat and the fear of venturing into the center of brightly lit arena (Bodnoff et al., 1988). The latency to feed in NSF test has been used as an index of anxiety-like behaviors (Santarelli et al., 2003). The NSF test was performed during a 5 min period, as described previously (Santarelli et al., 2001). In brief, the testing apparatus consisted of a plastic box (50 × 50 × 20 cm), the floor of which was covered with ∼2 cm wooden bedding. Twenty-four hours before behavioral testing, all the food was removed from the home cage. At the time of testing, a single pellet of food (regular chow) was placed on a white paper platform positioned in the center of the box. Each mouse was placed in a corner of the box, facing the corner, and a stopwatch was immediately started. The latency to eat (defined as the mouse sitting on its haunches, holding the pellet with its forepaws, and biting the pellet) was timed. Immediately after this test, the animal was transferred to its home cage, and the latency to eat and the amount of food consumed by the mouse in 15 min were measured, serving as a control for change in appetite as a possible confounding factor.
Open-field test.
The open-field is a widely used conflict procedure, in which the innate drive to explore a novel environment is opposed by the tendency to stay in protective areas, namely the outer fields flanked by walls. Thus, mice avoid the unsafe and aversive appearance of the inner fields and typically spend much more time in the outer than in the inner fields (Crawley, 1985). Open-field activity assay was performed at 24 h after the NSF test using the MotorMonitor System SF16R. The test arena was constructed of a plastic plate (56.13 × 56.13 cm) and divided into 256 squares by lines drawn on the floor of the plate. It was surrounded by a 35.18-cm-high plastic wall. The 64 squares in the center of the test arena were referred to as inner squares and the remaining ones as outer squares. Each mouse was placed onto a corner square of the arena, facing the corner, and allowed to freely explore the open field for 5 min per trial. During the period, the numbers of entered outer and inner squares were counted. An entry into a square was defined as having the two forelimbs in the square at one time. After each trial the plate was cleaned with 70% EtOH.
Elevated plus maze test.
Unconditioned anxiety-like behaviors were assessed at 24 h after the NSF test using an elevated plus maze (EPM) consisting of two open arms (30 × 5 cm), two enclosed arms (30 × 5 cm, with end and side walls 15 cm high), and a connecting central platform (5 × 5 cm). The maze was raised to a height 38.5 cm above the floor. Each mouse was placed in the intersection of the four arms of the maze, facing an open arm and allowed to explore freely for 5 min. An arm entry was defined as a mouse entering an arm of the maze with all four legs. General activity/exploration was evaluated using the total number of entries into the arms. Anxiety was assessed using time spent in open arms.
Culture of hippocampal neurons.
Hippocampi of P0 C57/BL/6 mice were removed and placed in HBSS without Ca2+ and Mg2+ (Invitrogen) containing 1 mm sodium pyruvate and 10 mm HEPES. Then the hippocampal tissues were dissociated in HBSS containing 0.125% trypsin solution for 10 min at 37°C. Subsequently, tissues were triturated by repeated passage through a constricted Pasteur pipette. The digestion was stopped with DMEM along with 10% heat-inactivated fetal bovine serum. The dispersed tissues were allowed to settle for 3 min. The supernatant was transferred to a fresh tube and centrifuged at 2000 rpm for 2 min. The pellet was resuspended in a neuron-defined culture medium, serum-free neurobasal medium (Invitrogen), supplemented with B-27, 0.5 mm l-glutamine, 20 IU/ml penicillin, and 20 IU/ml streptomycin. The cells were then plated onto six-well plates coated with poly-d-lysine (100 μg/ml) at 4 × 106 per well. Cell cultures were kept in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Half of the medium was replaced with fresh medium without glutamate every 2–3 d. The purity of neuronal cultures was determined by immunofluorescence using staining with antibody against β-tubulin (1:200; Millipore Bioscience Research Reagents), and the nuclei were stained for 15 min with Hoechst 33258 (1 μg · ml−1 in PBS). The purity of the neurons used in experiments was ∼95%.
nNOS immunofluorescence.
Fixed hippocampal neurons or brain sections were incubated with mouse anti-nNOS antibody (1:200; Millipore Bioscience Research Reagents) and binding was visualized with a Cy3-conjugated secondary antibody (1:200; Millipore Bioscience Research Reagents). Images of immunostained neurons in all groups were captured with a Zeiss Axio Cam MRC 5(D) camera mounted on Carl Zeiss Axio Observer A1 microscope under same conditions. The immunofluorescence of nNOS was analyzed using ImagePro (Media Cybernetics). For brain sections, fluorescence intensity in the hippocampus, hypothalamus, or amygdala was measured in a 650 × 505 μm2 area in each section. We randomly chose 10 nNOS-positive neurons in this area to measure brightness of staining. The brightness from sampled sections was averaged.
Western blot analysis.
Samples from cultured hippocampal neurons and hippocampal tissues of animals were prepared as described by our previous studies (Luo et al., 2007; Hu et al., 2008). The samples containing equivalent amounts of protein (20 μg) were applied to 8–12% [8% for nNOS, inducible NOS (iNOS), and endothelial NOS (eNOS); 12% for CREB and phosphorylated CREB (pCREB)] acrylamide denaturing gels (SDS-PAGE). The separated proteins were transferred onto nitrocellulose membranes overnight at 4°C. Blotting membranes were incubated with blocking solution [5% nonfat dried milk powder dissolved in TBST buffer (pH 7.5, 10 mm Tris-HCl, 150 mm NaCl, and 0.1% Tween 20)] for 1 h at room temperature, washed three times, and then incubated with rabbit antiphospho-CREB-ser133 (1:2000; Santa Cruz Biotechnology), rabbit anti-CREB (1:2000; Sigma-Aldrich), mouse anti-iNOS (1:2000; Santa Cruz Biotechnology), mouse anti-nNOS (1:1000; Millipore Bioscience Research Reagents), or mouse anti-eNOS (1:2000, Millipore Bioscience Research Reagents) in TBST overnight at 4°C. Internal control was performed using β-actin antibody (1:1000, Sigma-Aldrich). After several washes with TBST buffer, the membranes were incubated for 1 h with horseradish peroxidase-linked secondary antibody (1:10000). The membranes were then processed with enhanced chemiluminescence Western blotting detection reagents (Pierce). The films were scanned and densitometry was performed using the Quantity One image software (Bio-Rad). The relative level of the protein was quantified from the scanned films.
Reverse transcription-PCR.
Total RNA was extracted from the hippocampus using Trizol reagent according to the manufacturer's instructions (Sigma-Aldrich). The primers for nNOS and β-actin were as follows: nNOS: forward, 5′-TGT CCT ATA CAG CTT CCA GA-3′ and reverse, 5′-CAC GAT GTC ATA TTC CTC CA-3′; β-actin: forward, 5′-CAC GAT GGA GGG GCC GGA CTC ATC-3′ and reverse, 5′-TAA AGA CCT CTA TGC CAA CAC AGT-3′. PCR conditions were 30 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 45 s. PCR products were separated by electrophoresis through 1.5% agarose gel containing 0.5% μg/ml ethidium bromide and imaged using a BioDoc-IT imaging system (Bio-Rad); band intensities were determined using GS-710 calibrated imaging Densitometer (Bio-Rad). The mRNA for β-actin was included in the PCR mixture as a standard.
Statistical analysis.
Data are presented as means ± SEM. The significance of differences was determined using one-way ANOVAs followed by Scheffe's post hoc test. When two factors were assessed, the significance of differences was determined using two-way ANOVAs. Differences were considered significant when p < 0.05.
Results
5-HT1AR exerts negative control on nNOS expression
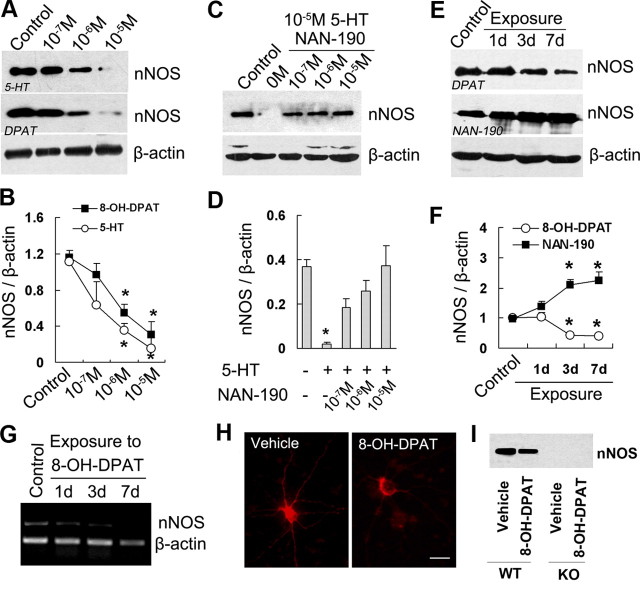
To investigate the possible link between nNOS and 5-HT1AR, we examined nNOS expression in the cultured hippocampal neurons exposed to 8-OH-DPAT (a 5-HT1AR-selective agonist) or 5-HT for 24 h by Western blotting. Both drugs reduced nNOS levels concentration-dependently (Fig. 1A,B). To determine whether 5-HT1AR activation is critical for the effect of 5-HT on nNOS expression, we incubated hippocampal neurons with 5-HT for 24 h in the presence of 0, 0.1, 1, or 10 μm NAN-190 (a 5-HT1AR-selective antagonist). As expected, NAN-190 abolished the effect of 5-HT thoroughly (Fig. 1C,D). To further assess nNOS expression in response to 5-HT1AR activation in vivo, we treated adult mice with 8-OH-DPAT or NAN-190 for 1, 3, or 7 d. Treatment with 8-OH-DPAT for 3 (F(3,9) = 9.76; p = 0.018) or 7 d (F(3,9) = 9.76; p = 0.009) decreased hippocampal nNOS expression, whereas treatment with NAN-190 for 3 (F(3,8) = 11.88; p = 0.024) or 7 d (F(3,8) = 11.88; p = 0.019) increased nNOS levels significantly (Fig. 1E,F). Similarly, we treated adult mice with 8-OH-DPAT for 1, 3, or 7 d and found that the drug reduced nNOS mRNA levels significantly at 3 and 7 d after treatment (Fig. 1G). In addition, we exposed hippocampal neurons to 10−5 m 8-OH-DPAT for 24 h and found that the drug decreased the immunofluorescence intensity of nNOS-positive neurons (Fig. 1H). To address the local effect of 8-OH-DPAT, we microinjected it (3.28 μg) into the hippocampus of WT or KO mice, measured hippocampal nNOS levels at 24 h after injections, and found that the drug reduced nNOS expression in WT mice (55.99 ± 4.86% of control; F(1,4) = 35.37; p = 0.004) (Fig. 1I).
Figure 1.
5-HT1AR exerts negative control on nNOS expression. A, B, Immunoblots of nNOS in the cultured hippocampal neurons incubated with 5-HT or 8-OH-DPAT for 24 h at the concentrations indicated. C, D, Immunoblots of nNOS in the cultured hippocampal neurons incubated with 10−5 m 5-HT for 24 h in the presence of NAN-190 at the concentrations indicated. E, F, Immunoblots of nNOS in the hippocampus of mice treated with 8-OH-DPAT or NAN-190 for 1, 3, or 7 d. G, Reverse transcription PCR examining mRNA levels of nNOS from the hippocampus of mice treated with 8-OH-DPAT or vehicle for 1, 3, and 7 d. H, Representative of nNOS immunofluorescence from the cultured hippocampal neurons incubated with 10−5 m 8-OH-DPAT or vehicle for 24 h. I, Immunoblots of nNOS in the hippocampus of KO or WT mice exposed to hippocampal 10−5 m 8-OH-DPAT microinjections for 24 h. Scale bar, 20 μm. In G–I, similar results were observed in each of four experiments. Error bars represent means ± SEM. n = 3–4; *p < 0.05 versus control. DPAT, 8-OH-DPAT.
Three isoforms of NOS exist and include nNOS, eNOS, and iNOS (Zhou and Zhu, 2009). Accordingly, we measured the protein levels of iNOS and eNOS in the hippocampus of adult mice treated with 8-OH-DPAT for 1, 3, or 7 d. Neither iNOS nor eNOS was changed by the drug (p > 0.05) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Together, these findings suggest that 5-HT1AR exerts negative control on nNOS expression selectively.
Additionally, we observed the distribution of nNOS-positive neurons in the hippocampus, hypothalamus, and amygdala, where postsynaptic 5-HT1AR is richly expressed (Gross et al., 2000), and found that most nNOS-expressing neurons in the hippocampus are strongly positive, whereas most nNOS-expressing neurons in the hypothalamus are weakly positive (65.76 ± 2.52% of hippocampus; F(2,27) = 66.48; p < 0.0001) or amygdala (49.65 ± 1.91% of hippocampus; F(2,27) = 66.48; p < 0.0001) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
nNOS plays a role in mediating anxiety-related behaviors
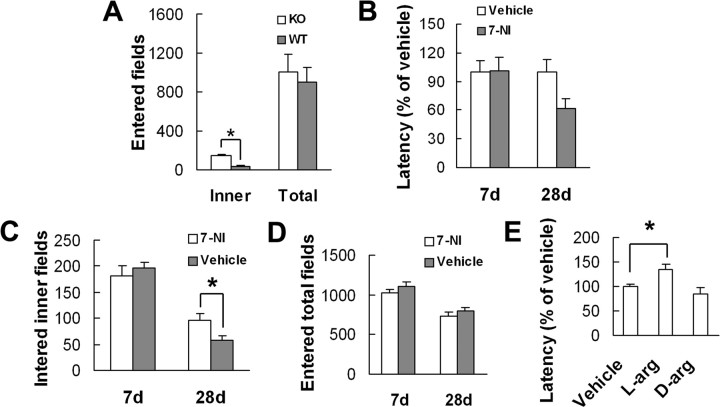
To determine whether null mutant mice lacking nNOS gene (nNOS KO mice) have altered anxiety-related behaviors, we compared latency to feed in the NSF test in KO and in WT mice. As expected, KO mice displayed a lower latency than their littermate controls to begin feeding in novel environment (54.67 ± 5.85% of WT mice; F(1,16) = 10.98; p = 0.004), indicating an anxiolytic-like phenotype. As KO and WT mice had similar latency to feed in home cage (F(1,16) = 0.50; p = 0.490) and amount of home cage food consumption (F(1,16) = 3.75; p = 0.071) (supplemental Fig. 3A,B, available at www.jneurosci.org as supplemental material), it is unlikely that alterations in appetite account for the changes in latency to feed in novel environment in KO mice. Consistently, KO mice in the open-field test crossed the inner fields of the arena significantly more frequently as compared with WT mice (F(1,18) = 88.46; p < 0.0001), displaying a reduced anxiety-like behavior. This did not result from a difference in spontaneous locomotion or exploratory behavior, since the total number of all entered fields was similar between the groups (F(1,18) = 0.20; p < 0.657) (Fig. 2A).
Figure 2.
nNOS is implicated in anxiety-related behavior. A, The number of entered total fields and of entered inner fields for nNOS KO and WT mice in the open field (n = 10). B, The latency to feed in the NSF test in the mice treated with 7-NI (30 mg/kg, i.p.) or vehicle for 7 d (n = 11–12) or 28 d (n = 10–12). C, D, The number of entered inner fields (C) or entered total fields (D) for the mice treated with 7-NI (30 mg/kg, i.p.) or vehicle for 7 or 28 d (n = 10–11). E, The latency to feed in the NSF test in l-arginine (L-arg)- and d-arginine (D-arg)-treated mice (n = 10–12). Error bars represent means ± SEM. *p < 0.05.
To further ascertain the effects of nNOS inhibition on anxiety-related behaviors, we treated mice with 7-NI, a selective nNOS inhibitor, for 7 d before performing the NSF test. This treatment failed to change the latency to feed in novel environment (F(1,21) = 0.01; p = 0.905) (Fig. 2B). Our previous study showed that nNOS is involved in chronic mild stress-induced depression by a delay action mechanism (Zhou et al., 2007). To examine whether nNOS inhibitor has effect on anxiety-related behaviors in a delay action manner, we administered 7-NI for 28 d before performing the NSF test. Interestingly, this treatment produced a significantly decreased latency to feed in novel environment (F(1,20) = 5.08; p = 0.036) as compared with vehicle group (Fig. 2B), and did not change the latency to feed in home cage (F(1,20) = 1.27; p = 0.274) or the amount of home cage food consumption (F(1,20) = 0.23; p = 0.638) (supplemental Fig. 3C,D, available at www.jneurosci.org as supplemental material). Also, mice treated with 7-NI for 28 d (F(1,19) = 4.89; p = 0.039), but not those treated for for 7 d (F(1,19) = 0.46; p = 0.505), crossed the inner fields of the arena significantly more frequently, as compared with vehicle group (Fig. 2C). The total number of all entered fields was similar between mice treated for 7 d (F(1,19) = 1.39; p = 0.253) and mice treated for 28 d (F(1,19) = 0.74; p = 0.400) (Fig. 2D).
To strengthen the implication of nNOS in NSF behavior, we treated mice with l-arginine (a unique nitric oxide synthase substrate) or d-arginine for 28 d before performing the test. As shown in Figure 2E, l-arginine produced a significantly prolonged latency to feed in novel environment (F(2,31) = 7.89; p = 0.034), whereas d-arginine was ineffective (F(2,31) = 7.89; p = 0.485). Considering that l-arginine increased home cage food consumption (F(2,31) = 4.02; p = 0.029) (supplemental Fig. 3E, available at www.jneurosci.org as supplemental material), the increase in the latency to feed in novel environment was not likely due to decreased appetite. Together, these results indicate that nNOS plays an important role in mediating anxiety-related behaviors.
nNOS is required for the behavioral effects of 5-HT1AR agonist and antagonist
To determine whether nNOS is required for the effect of 5-HT1AR agonist on anxiety-related behaviors, we treated KO and WT mice with 8-OH-DPAT or vehicle for 28 d before performing the NSF test. The treatment significantly decreased the latency to feed in novel environment in WT mice (F(1,33) = 8.46; p = 0.0064) but was ineffective in KO mice (F(1,33) = 0.04; p = 0.8392), and vehicle-treated KO mice displayed a lower latency than vehicle-treated WT mice did (F(1,33) = 4.68; p = 0.0379) (Fig. 3A). The drug did not change the latency to feed in home cage both in KO (F(1,33) = 0.06; p = 0.8013) and in WT mice (F(1,33) = 0.08; p = 0.7802) (supplemental Fig. 4A, available at www.jneurosci.org as supplemental material). Also, the drug had no effect on the amount of home cage food consumption both in KO mice (F(1,33) = 0.00; p = 0.9700) and in WT mice (F(1,33) = 0.42; p = 0.5223) compared with vehicle (supplemental Fig. 4B, available at www.jneurosci.org as supplemental material). In addition, the latencies for untreated KO mice in Figures 2A and 3A were almost identical, suggesting a replicated behavioral phenotype in separate cohorts of KO mice. In WT mice, 8-OH-DPAT caused a significant increase in the number of entered inner fields, as compared with vehicle (F(1,36) = 10.07; p = 0.0031). In KO mice, however, the drug was ineffective (F(1,36) = 0.18; p = 0.6724) (Fig. 3B). The total number of all entered fields was similar between the groups both in KO (F(1,36) = 0.43; p = 0.5142) and in WT (F(1,36) = 0.09; p = 0.7598) mice (Fig. 3C).
Figure 3.
nNOS is required for the effects of 5-HT1AR agonist and antagonist on anxiety-related behavior. A, The latency to feed in the NSF test in nNOS KO and WT mice treated with 8-OH-DPAT or vehicle for 28 d (n = 9–10). B, C, The number of entered inner fields (B) and of entered total fields (C) for nNOS KO and WT mice treated with 8-OH-DPAT or vehicle for 28 d in the open field (n = 10). D, The latency to feed in the NSF test in mice treated with NAN-190 or 7-NI or the combination of the two drugs for 28 d (n = 9–10). E, The latency to feed in the NSF test in the mice treated with NAN-190 or 7-NI or the combination of two drugs for 28 d (n = 9–10). Error bars represent means ± SEM. *p < 0.05, versus vehicle; #p < 0.05, versus NAN-190.
Next, we investigated the effect of 5-HT1AR-selective antagonist on NSF behavior in KO and WT mice. As shown in Figure 3D, treatment with NAN-190 for 28 d caused a significant increase in the latency to feed in novel environment in WT mice, as compared with vehicle (F(1,34) = 12.40; p = 0.0012), suggesting an anxiogenic-like behavior. In KO mice, however, no significantly different latency was observed between groups (F(1,34) = 0.02; p = 0.9009). NAN-190 did not change the latency to feed in home cage either in KO (F(1,34) = 0.31; p = 0.5831) or in WT (F(1,34) = 0.01; p = 0.9165) mice (supplemental Fig. 4C, available at www.jneurosci.org as supplemental material). Also, the drug had no effect on the amount of home cage food consumption either in KO (F(1,34) = 0.89; p = 0.3530) or in WT (F(1,34) = 0.16; p = 0.6872) mice (supplemental Fig. 4D, available at www.jneurosci.org as supplemental material).
To further address the implication of nNOS in 5-HT1AR-medicated NSF behavior, we treated mice with NAN-190, 7-NI, or both in combination for 28 d before performing the test. As shown in Figure 3E, NAN-190 prolonged the latency to feed in novel environment compared with vehicle (F(1,35) = 19.38; p = 0.0001). Pretreatment with 7-NI abolished the effect of NAN-190 on the NSF behavior (F(1,35) = 30.14; p < 0.0001) compared with NAN-190 alone. These treatments did not alter the latency to feed in home cage (NAN-190 vs vehicle, F(1,35) = 0.19, p = 0.6692; 7-NI vs vehicle, F(1,35) = 0.23, p = 0.6326; 7-NI/NAN-190 vs NAN-190, F(1,35) = 0.18, p = 0.6762) or the amount of home cage food consumption (NAN-190 vs vehicle, F(1,35) = 0.02, p = 0.8882; 7-NI vs vehicle, F(1,35) = 0.05, p = 0.8228; 7-NI/NAN-190 vs NAN-190, F(1,35) = 0.25, p = 0.6215) (supplemental Fig. 5, available at www.jneurosci.org as supplemental material). These data suggest that nNOS is required not only for the anxiolytic effect of 5-HT1AR activation but also for the anxiogenic effect of 5-HT1AR dysfunction.
To address the anatomical specificity of pharmacological manipulations, we infused 8-OH-DPAT into the left hippocampus of mice using 14 d Alzet osmotic minipump and performed the NSF test and EPM 7 d after the removal of the minipump. In the NSF test, this infusion significantly decreased the latency to feed in novel environment (60.25 ± 10.03% of vehicle; F(1,17) = 6.4; p = 0.022), and did not change the latency to feed in home cage or the amount of home cage food consumption (data not shown). Consistently, the EPM test revealed that 8-OH-DPAT significantly increased time spent in open arms (70.6 ± 8.03 vs 37.78 ± 5.16 s; F(1,17) = 6.12; p = 0.024), but did not change the total number of entries in the arms (15.3 ± 1.55 vs 17.44 ± 1.63; F(1,17) = 1.49; p = 0.240) compared with vehicle. Moreover, we delivered 8-OH-DPAT into the bilateral hippocampi by microinjections and performed the NSF test and EPM test at 7 or 21 d after injections. Consistent with the experiment using the osmotic minipump, 8-OH-DPAT-treated mice exhibited anxiolytic-like behaviors at 21 d after treatment. However, hippocampal 8-OH-DPAT microinjection had no effect in either the NSF or the EPM tests at 7 d after treatment (supplemental Fig. 6, available at www.jneurosci.org as supplemental material).
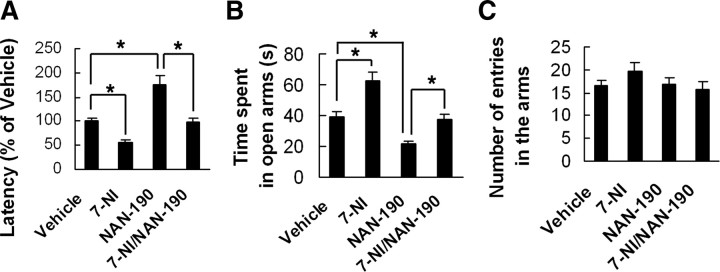
To determine whether hippocampal nNOS is essential for the role of 5-HT1AR, we microinjected 7-NI (16.31 μg), NAN-190 (4.74 μg), or both in combination (NAN-190 was injected at 30 min after 7-NI injection) into the bilateral hippocampi of adult mice. The NSF test and EPM test were performed at 21 d after treatments. In the NSF test, NAN-190 significantly increased (F(1,39) = 27.22; p < 0.0001), whereas 7-NI decreased the latency to feed in novel environment compared with vehicle (F(1,39) = 8.74; p = 0.0053). Pretreatment with 7-NI neutralized the anxiogenic effect of NAN-190 (F(1,39) = 30.19; p < 0.0001) (Fig. 4A). These microinjections did not change the latency to feed in home cage or the amount of home cage food consumption (data not shown). In the EPM test, NAN-190 significantly decreased (F(1,38) = 11.55; p = 0.0016), whereas 7-NI increased time spent in open arms (F(1,38) = 20.68; p = 0.0001). Pretreatment with 7-NI neutralized the effects of NAN-190 on time spent in open arms (F(1,38) = 9.61; p = 0.0036) (Fig. 4B). NAN-190, 7-NI, or both in combination did not affect the total number of entries in the arms (p > 0.05) (Fig. 4C). These data suggest a significant implication of the hippocampal nNOS in 5-HT1AR-associated anxiety-related behaviors.
Figure 4.
Neuronal NOS inhibition abolishes the anxiogenic-like effect of 5-HT1AR dysfunction. NAN-190 (4.74 μg), 7-NI (16.31 μg), or both in combination (NAN-190 was injected at 30 min after 7-NI injection) was microinjected into the bilateral hippocampi of adult mice. A–C, The latency to feed in the NSF test (A) and time spent in open arms (B) and number of entries in the arms (C) in the EPM test were measured at 21 d after treatments. Error bars represent means ± SEM. n = 9∼12; *p < 0.05.
nNOS is required for the behavioral effect of SSRIs but not of noradrenaline reuptake inhibitors
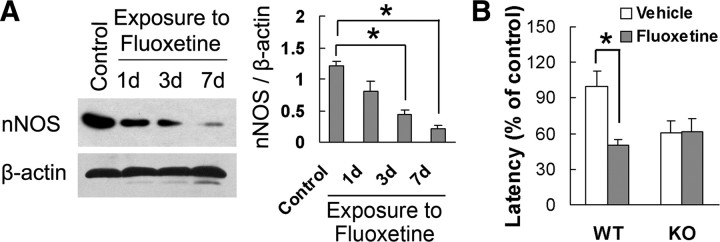
The activation of 5-HT1AR is critical for the anxiolytic effects of SSRIs (Santarelli et al., 2003), the most widely prescribed class of anxiolytic drugs (Gorman, 2002). To determine whether nNOS is also implicated in the effects of SSRIs, we examined nNOS, iNOS, and eNOS expression in the hippocampus of adult mice treated with fluoxetine for 1, 3, or 7 d. The drug significantly decreased hippocampal nNOS levels after treatment for 3 d (F(3,8) = 21.51; p = 0.003) or 7 d (F(3,8) = 21.51; p = 0.001) (Fig. 5A), and had no effect on iNOS or eNOS (p > 0.05) (supplemental Fig. 7, available at www.jneurosci.org as supplemental material). Next, we investigated the effect of fluoxetine on NSF behavior in KO and WT mice. Treatment with the drug for 28 d significantly shortened the latency to feed in novel environment in WT mice (F(1,36) = 11.97; p = 0.0014) but was ineffective in KO mice (F(1,36) = 0.00; p = 0.9802), as compared with vehicle group (Fig. 5B). The treatment did not change the latency to feed in home cage (for KO mice, F(1,36) = 0.54, p = 0.4685; for WT mice, F(1,36) = 0.00, p = 0.9735) and the amount of home cage food consumption (for KO mice, F(1,36) = 2.66, p = 0.1118; for WT mice, F(1,36) = 0.42, p = 0.5226) (supplemental Fig. 8, available at www.jneurosci.org as supplemental material).
Figure 5.
nNOS is required for the effects of SSRI on anxiety-related behavior. A, Immunoblots of nNOS in the hippocampus of mice treated with fluoxetine for 1, 3, or 7 d (n = 3). B, The latency to feed in the NSF test in nNOS KO and WT mice treated with fluoxetine or vehicle for 28 d (n = 9∼12). Error bars represent means ± SEM. *p < 0.05.
The noradrenergic system is another monoaminergic system implicated in anxiety (Wong and Licinio, 2001; Santarelli et al., 2003); we therefore assessed whether nNOS is associated with the behavioral effect of noradrenaline-reuptake inhibitors (NRIs). We examined nNOS expression in the cultured hippocampal neurons exposed to 0, 0.1, 1, or 10 μm noradrenalin for 24 h. The drug had no effect on nNOS expression (F(3,8) = 0.59, p > 0.05, any group vs vehicle group) (supplemental Fig. 9A, available at www.jneurosci.org as supplemental material). Next, we treated adult mice with imipramine or desipramine, two noradrenaline reuptake inhibitors, for 7 d and examined nNOS expression in the hippocampus tissues. Neither imipramine (F(1,4) = 0.05; p = 0.841) nor desipramine (F(1,4) = 0.09; p = 0.782) altered nNOS level compared with vehicle (supplemental Fig. 9B, available at www.jneurosci.org as supplemental material). Finally, we investigated the effect of imipramine on NSF behavior in KO and WT mice. Interestingly, treatment with imipramine for 28 d significantly shortened latency to feed in novel environment both in WT (F(1,28) = 13.83; p = 0.0009) and KO (F(1,28) = 5.13; p = 0.0315) mice, as compared with vehicle (supplemental Fig. 9C, available at www.jneurosci.org as supplemental material). The drug had no effect on the latency to feed in home cage (for WT mice, F(1,28) = 0.00, p = 0.9579; for nNOS KO mice, F(1,28) = 0.34, p = 0.5659) (supplemental Fig. 10A, available at www.jneurosci.org as supplemental material) or the amount of home cage food consumption (for WT mice, F(1,28) = 0.87, p = 0.3596; for KO mice, F(1,28) = 0.17, p = 0.6790) (supplemental Fig. 10B, available at www.jneurosci.org as supplemental material).
nNOS is required for 5-HT1AR-mediated CREB phosphorylation
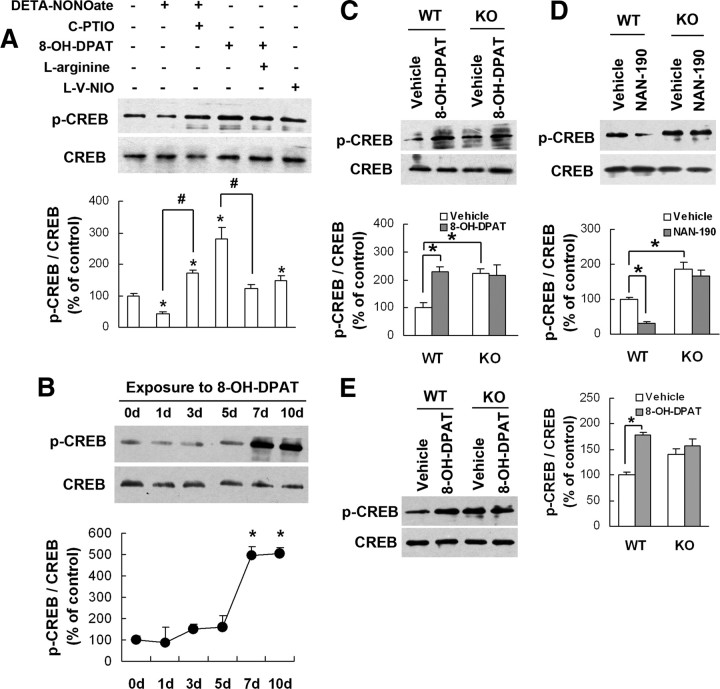
CREB knock-out mice exhibited increase in anxiety-like behaviors (Valverde et al., 2004). Neuronal NOS controls CREB-mediated gene expression in neurons (Riccio et al., 2006). We thus hypothesized that 5-HT1AR affects CREB activity via nNOS. To address this hypothesis, we exposed hippocampal neurons to 100 μm l-V-NIO (a selective nNOS inhibitor), 100 μm DETA-NONOate (a NO donor) alone or combined with 20 μm C-PTIO (a NO scavenger), or 10 μm 8-OH-DPAT alone or combined with 100 μm l-arginine for 24 h. As shown in Figure 6A, DETA-NONOate significantly reduced pCREB levels (42.7 ± 7.9% of control; F(1,6) = 17.58; p = 0.0057; vs vehicle), and C-PTIO reversed the effect of DETA-NONOate (171.2 ± 11.7% of control; F(1,6) = 88.39; p = 0.0001; DETA-NONOate vs DETA-NONOate/C-PTIO). In contrast, 8-OH-DPAT remarkably increased pCREB levels (278.5 ± 39.6% of control; F(1,6) = 26.42; p = 0.0021; vs vehicle), and l-arginine reversed the effect of 8-OH-DPAT (121.7 ± 12.5% of control; F(1,6) = 20.38; p = 0.0040; 8-OH-DPAT vs 8-OH-DPAT/l-arginine). Furthermore, l-V-NIO also increased pCREB levels (149.1 ± 15.1% of control; F(1,4) = 7.78; p = 0.049; vs vehicle). To determine whether NRIs change CREB activation, we examined pCREB in the hippocampal neurons exposed to 0, 0.1, 1, or 10 μm noradrenalin for 24 h and found that it had no effect (F(3,8) = 0.81; p > 0.05; any group vs vehicle) (supplemental Fig. 11, available at www.jneurosci.org as supplemental material).
Figure 6.
nNOS is required for the 5-HT1AR receptor-mediated CREB phosphorylation. A, Immunoblots of pCREB in the cultured hippocampal neurons incubated with drugs indicated for 24 h (n = 3). B, Immunoblots of pCREB in the hippocampus of adult mice treated with 8-OH-DPAT for 0, 1, 3, 5, 7, or 10 d (n = 3). C, Immunoblots of pCREB in the hippocampus of nNOS KO and WT mice treated with 8-OH-DPAT or vehicle for 10 d (n = 3). D, Immunoblots of pCREB in the hippocampus of nNOS KO and WT mice treated with NAN-190 or vehicle for 14 d (n = 3). E, Immunoblots of pCREB in the hippocampus of nNOS KO or WT mice exposed to hippocampal 10−5 m 8-OH-DPAT microinjections for 24 h (n = 3). Error bars represent means ± SEM. *p < 0.05 (in A and B vs control); #p < 0.05.
Next, we administered 8-OH-DPAT in adult mice for 1, 3, 5, 7, or 10 d and found that it increased pCREB after treating for 7 d (495.8 ± 40.0% of control; F(5,12) = 19.32; p = 0.002) or 10 d (504.3 ± 29.7% of control; F(5,12) = 19.32; p = 0.001) (Fig. 6B). Finally, we treated WT and KO mice with 8-OH-DPAT or NAN-190 for 14 d. As shown in Figure 6, C and D, 8-OH-DPAT markedly increased pCREB (230.7 ± 15.2% of control; F(1,8) = 15.83; p = 0.0041), whereas NAN-190 decreased pCREB in WT mice (30.6 ± 5.95% of control; F(1,8) = 12.52; p = 0.0076), and they were ineffective in KO mice (for 8-OH-DPAT, F(1,8) = 0.05, p = 0.8212; for NAN-190, F(1,8) = 0.86, p = 0.3806). To address the anatomical specificity of drugs tested, we microinjected 8-OH-DPAT (3.28 μg) into the hippocampus of KO and WT mice and measured hippocampal pCREB at 24 h after injections. As shown in Figure 6E, 8-OH-DPAT significantly increased pCREB in WT mice (178 ± 5.0% of control; F(1,8) = 33.85; p = 0.0004) but not in KO mice (F(1,8) = 1.74; p = 0.2240). Together, our data suggest that nNOS is required for 5-HT1AR-mediated CREB phosphorylation in the hippocampus.
Hippocampal CREB phosphorylation is essential for behavioral effects of nNOS
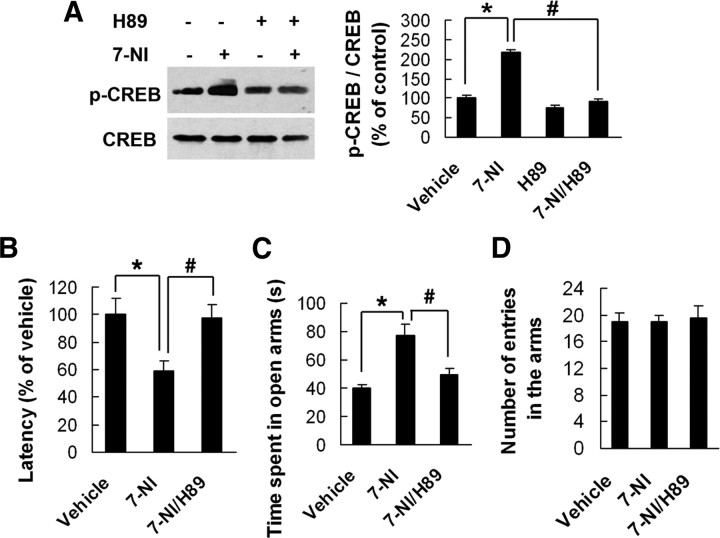
To determine whether hippocampal CREB activity is essential for the role of nNOS in anxiety-related behaviors, we delivered H89 (5.19 μg), a PKA inhibitor, or 7-NI (16.31 μg), or both in combination (7-NI was injected at 30 min after H89 injection) into the hippocampus of adult mice and measured hippocampal pCREB at 24 h after microinjections. As shown in Figure 7A, 7-NI markedly increased hippocampal pCREB level compared with vehicle (F(1,8) = 150.42; p < 0.0001). The 7-NI-induced increase in hippocampal pCREB was neutralized by H89 pretreatment (F(1,8) = 171.88; p < 0.0001).
Figure 7.
Hippocampal CREB phosphorylation is essential for behavioral effects of nNOS. A, Immunoblots of pCREB in the hippocampus of adult mice exposed to hippocampal 7-NI, H89, or both in combination microinjections for 24 h (n = 3). B–D, PKA inhibitor H89 abolishes behavioral effects of nNOS inhibitor 7-NI. H89 (10−5 m) was microinjected into bilateral hippocampi of adult mice (for 3 times at 7 d intervals), and 7-NI was administrated (30 mg/kg, i.p.) for 21 d beginning immediately after first H89 injection. The latency to feed in the NSF test (B) and time spent in open arms (C) and number of entries in the arms (D) in the EPM test were measured at 24 h after last 7-NI injection. Error bars represent means ± SEM. n = 9∼10 in B–D. *p < 0.05 versus vehicle; #p < 0.05 versus 7-NI group.
Next, we delivered H89 into bilateral hippocampi of adult mice (5.19 μg three times at 7 d intervals). We treated these mice with systemic 7-NI (i.p.) for 21 d beginning immediately after first H89 injection and performed the NSF and EPM tests at 24 h after last 7-NI injection. In the NSF test, 7-NI significantly reduced the latency to feed in novel environment (F(1,25) = 9.15; p = 0.0057), and hippocampal H89 microinjection abolished the anxiolytic-like effect of 7-NI (F(1,25) = 8.00; p = 0.0091) (Fig. 7B). The latency to feed in home cage and the amount of home cage food consumption in all groups were similar (data not shown). Similarly, the EPM test revealed that 7-NI significantly increased time spent in open arms (F(1,25) = 19.98; p = 0.0001) and hippocampal H89 microinjection abolished the effects of 7-NI (F(1,25) = 11.08; p = 0.0027) (Fig. 7C). Neither 7-NI alone nor its combination with H89 changed the total number of entries in the arms (Fig. 7D). These results suggest that hippocampal CREB phosphorylation is essential for behavioral effects of nNOS.
Discussion
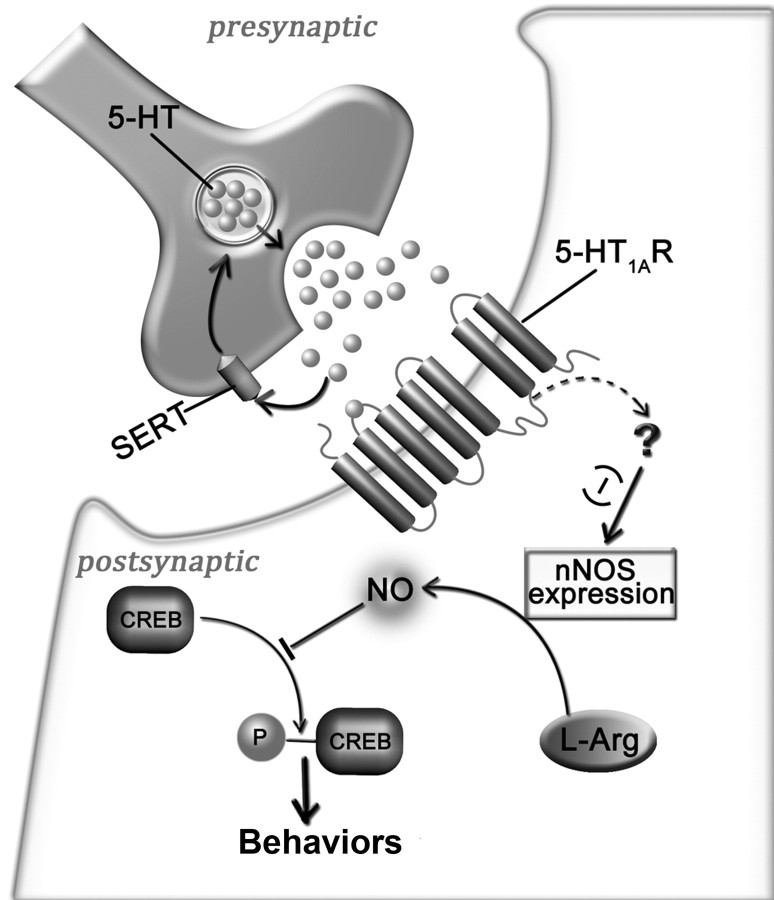
In the present study, we have identified a signaling cascade whereby 5-HT1AR regulates anxiety-related behaviors (Fig. 8). Serotonin, acting via 5-HT1AR, suppresses nNOS expression in the hippocampus. This modification leads to CREB activation, thereby modulating anxiety-related behaviors.
Figure 8.
A model of signal pathway whereby 5-HT1AR regulates anxiety-related behavior. Serotonin-5-HT1AR stimulation downregulates nNOS expression via an unknown mechanism and leads to a decrease in NO, a free radical that exerts a negative control on CREB phosphorylation, thus influencing anxiety-related behavior. (−), Negative regulation; SERT, serotonin transporter, which reuptakes 5-HT in synaptic cleft; ?, unknown mechanism; L-arg, l-arginine.
The serotonergic system represents a major common pathway for anxiety-related disorders (Wu et al., 2008). In particular, SSRIs are the first-line compounds for clinical treatment of anxiety-related disorders (Gorman, 2002). SSRI-induced increases in 5-HT are thought to act at all of the at least 15 types of mammalian 5-HT receptors (Hoyer et al., 1994). However, 5-HT1AR activation is critical for the anxiolytic effects of SSRIs (Santarelli et al., 2003), suggesting its key role in modulating anxiety-related behaviors. Indeed, 5-HT1AR agonists have been clinically used as anxiolytic drugs (Gorman, 2002). Knock-out of 5-HT1AR leads to an anxiogenic phenotype (Gingrich and Hen, 2001; Olivier et al., 2001; Santarelli et al., 2003). Tissue-specific expression of 5-HT1AR in the hippocampus and cortex of 5-HT1A KO mice recovers wild-type phenotype (Gross et al., 2002). Interestingly, our findings showed that nNOS was required not only for the anxiolytic effects of 5-HT1AR agonist and SSRIs, but also for the anxiogenic effects of 5-HT1AR antagonist, indicating that nNOS may serve as a downstream signaling enzyme of 5-HT1AR. Further support for this hypothesis comes from the fact that 5-HT1AR agonist and SSRIs downregulated nNOS expression, whereas 5-HT1AR antagonist upregulated nNOS expression in vitro and in vivo. Although chronic treatment with antidepressants that act via the noradrenergic mechanisms also produces anxiolytic effects (Santarelli et al., 2003), we found that that noradrenalin, imipramine, and desipramine did not influence nNOS expression and that deletion of nNOS did not alter the behavioral effects of imipramine, revealing a noradrenergic system independent of nNOS. Our findings are consistent with the notion that serotonin- and norepinephrine-enhancing antidepressants act via independent molecular pathways (Santarelli et al., 2003).
NO has multiple functions in nervous systems and has been implicated in the regulation of various behavioral, cognitive, and emotional processes (Zhou and Zhu, 2009). Neuronal NOS enriched throughout the limbic system (Bredt et al., 1991; Dawson et al., 1991) is appropriately positioned to play a prominent role in regulating emotional behaviors and plays an important role in normal brain 5-HT function, especially in the postsynaptic 5-HT1A and/or 5-HT1B receptor function (Chiavegatto et al., 2001). Our previous study showed that nNOS is essential for chronic stress-induced depression in mice (Zhou et al., 2007). Very recently, several studies have indicated that NOS is involved in anxiety-related behaviors (Miguel and Nunes-de-Souza, 2008; Workman et al., 2008). In the present study, nNOS KO mice exhibited a markedly decreased latency to feed in NSF test, suggesting an anxiolytic-like phenotype. The behavioral phenotype is likely due to the loss of nNOS, rather than a physiological compensation in response to deficient nNOS, because nNOS inhibitor produced a significant anxiolytic-like effect also. Since nNOS expression was substantially regulated by 5-HT1AR, the behavioral effects of nNOS inhibition may possibly explain how 5-HT1AR can regulate anxiety-related behaviors.
What might be the downstream molecular mechanisms underlying the role of 5-HT1A-nNOS pathway in the modulation of anxiety-related behaviors? We propose that CREB, a well known nuclear transcription factor, is the target of nNOS for its roles in modulating anxiety-like behaviors in several paradigms (Valverde et al., 2004). In the present study, 5-HT1AR agonist promoted CREB phosphorylation, whereas 5-HT1AR antagonist reduced CREB phosphorylation in vitro and in vivo, suggesting a link between 5-HT1AR activation and CREB phosphorylation. Recently, it has been demonstrated that NO pathway controls CREB-mediated gene expression in neurons (Riccio et al., 2006). Consistent with our previous findings that nNOS inhibition elevated hippocampal pCREB levels in vivo (Zhu et al., 2006; Luo et al., 2007), we showed here that nNOS-derived NO suppressed CREB phosphorylation in vitro, indicating an implication of nNOS in CREB phosphorylation. Because deletion of nNOS abolished the 5-HT1AR-mediated CREB activation and behaviors and, more importantly, CREB phosphorylation was essential for the behavioral effect of nNOS inhibition, it is very likely that CREB activation is critical for the roles of 5-HT1AR-nNOS pathway. Moreover, the data that noradrenalin did not change CREB phosphorylation further support the notion that 5-HT-acting drugs may function independently of norepinephrine-enhancing drugs.
The hippocampus is one of several limbic structures that have been associated with the modulation of emotional behaviors (McNaughton, 1997; Kempermann, 2002; Wu et al., 2008), with special emphasis on anxiety-related behaviors (Bannerman et al., 2004). The hippocampus is thought to control behavioral inhibition under conditions of conflict, when, for example, a novel, but potentially threatening, object or environment raises a conflict between approach and avoidance (McNaughton, 2006). Consequently, lesions of the hippocampus or local exposures to drugs lead to altered behavior in rodent models of anxiety (Menard and Treit, 2001; Deacon et al., 2002; Degroot and Treit, 2002).
Serotonin 1A receptor is expressed in two distinct neuronal populations in the brain: as an autoreceptor on serotonergic neurons in the raphe nuclei of the brainstem and as a heteroreceptor on nonserotonergic neurons in forebrain structures, primarily the hippocampus, septum, and cortex (Gross et al., 2000). In the hippocampus, 5-HT1AR subtype is predominantly postsynaptic to 5-HT neurons (Fairchild et al., 2003; Ogren et al., 2008). Mice lacking 5-HT1A receptor show increased anxiety-like behavior in a variety of conflict tests. Expression of 5-HT1A receptor, primarily in the hippocampus, but not in the raphe nuclei, is sufficient to rescue the anxiety-like phenotype of the knock-out mice (Gross et al., 2002). In the present study, hippocampal 5-HT1AR activation produced anxiolytic-like effects, whereas hippocampal 5-HT1AR dysfunction showed anxiogenic-like effects. Together, these findings indicate that the hippocampal postsynaptic 5-HT1AR is crucial for modulating anxiety-related behavior. Moreover, hippocampal nNOS inhibition produced anxiolytic-like effects and abolished the anxiogenic effects of 5-HT1AR antagonist. Thus, serotonin may regulate anxiety-related behaviors via 5-HT1AR-nNOS-CREB pathway in the hippocampus. Because both 5-HT1AR (Gross et al., 2000) and nNOS are expressed in the amygdala and the hypothalamus too, nNOS in other areas of the brain may also participate in the regulation of anxiety-related behaviors.
In summary, the present results demonstrate that 5-HT1AR modulate anxiety-related behaviors by suppressing nNOS expression and thereby promoting CREB activation in the hippocampus.
Footnotes
This work was supported by grants from National Natural Science Foundation of China (30870900), Natural Science Foundation of Jiangsu Province (BK2007728), and Specialized Research Fund for the Doctoral Program of Higher Education (200803120008) (all to D.Y.Z.). We thank S. M. Duan for helpful discussions and suggestions and L. Zhou and B. Wang for their help.
References
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus–memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Rawlins JN. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav Neurosci. 2002;116:494–497. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- Degroot A, Treit D. Dorsal and ventral hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Brain Res. 2002;949:60–70. doi: 10.1016/s0006-8993(02)02965-7. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Leitch MM, Ingram CD. Acute and chronic effects of corticosterone on 5-HT1A receptormediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology. 2003;45:925–934. doi: 10.1016/s0028-3908(03)00269-7. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology. 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Treatment of generalized anxiety disorder. J Clin Psychiatry. 2002;63(Suppl 8):17–23. [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Hawgood J, De Leo D. Anxiety disorders and suicidal behaviour: an update. Curr Opin Psychiatry. 2008;21:51–64. doi: 10.1097/YCO.0b013e3282f2309d. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors of 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hu M, Sun YJ, Zhou QG, Chen L, Hu Y, Luo CX, Wu JY, Xu JS, Li LX, Zhu DY. Negative regulation of neurogenesis and spatial memory by NR2B-containing NMDA receptors. J Neurochem. 2008;106:1900–1913. doi: 10.1111/j.1471-4159.2008.05554.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Regulation of adult hippocampal neurogenesis - implications for novel theories of major depression. Bipolar Disord. 2002;4:17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- Khan A, Leventhal RM, Khan S, Brown WA. Suicide risk in patients with anxiety disorders: a meta-analysis of the FDA database. J Affect Disord. 2002;68:183–190. doi: 10.1016/s0165-0327(01)00354-8. [DOI] [PubMed] [Google Scholar]
- Luo CX, Zhu XJ, Zhou QG, Wang B, Wang W, Cai HH, Sun YJ, Hu M, Jiang J, Hua Y, Han X, Zhu DY. Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by upregulating inducible nitric oxide synthase expression. J Neurochem. 2007;103:1872–1882. doi: 10.1111/j.1471-4159.2007.04915.x. [DOI] [PubMed] [Google Scholar]
- McNaughton N. Cognitive dysfunction resulting from hippocampal hyperactivity–a possible cause of anxiety disorder? Pharmacol Biochem Behav. 1997;56:603–611. doi: 10.1016/s0091-3057(96)00419-4. [DOI] [PubMed] [Google Scholar]
- McNaughton N. The role of the subiculum within the behavioural inhibition system. Behav Brain Res. 2006;174:232–250. doi: 10.1016/j.bbr.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D. The anxiolytic effects of intra-hippocampal midazolam are antagonized by intra-septal l-glutamate. Brain Res. 2001;888:163–166. doi: 10.1016/s0006-8993(00)03046-8. [DOI] [PubMed] [Google Scholar]
- Miguel TT, Nunes-de-Souza RL. Anxiogenic-like effects induced by NMDA receptor activation are prevented by inhibition of neuronal nitric oxide synthase in the periaqueductal gray in mice. Brain Res. 2008;1240:39–46. doi: 10.1016/j.brainres.2008.08.068. [DOI] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Eriksson TM, Elvander-Tottie E, D'Addario C, Ekström JC, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Olivier B, Pattij T, Wood SJ, Oosting R, Sarnyai Z, Toth M. The 5-HT(1A) receptor knockout mouse and anxiety. Behav Pharmacol. 2001;12:439–450. doi: 10.1097/00008877-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Placidi GP, Oquendo MA, Malone KM, Brodsky B, Ellis SP, Mann JJ. Anxiety in major depression: relationship to suicide attempts. Am J Psychiatry. 2000;157:1614–1618. doi: 10.1176/appi.ajp.157.10.1614. [DOI] [PubMed] [Google Scholar]
- Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R, Heath MJ. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc Natl Acad Sci U S A. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, de Graaf R, Asmundson GJ, ten Have M, Stein MB. Anxiety disorders and risk for suicidal ideation and suicide attempts. Arch Gen Psychiatry. 2005;62:1249–1257. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Valverde O, Mantamadiotis T, Torrecilla M, Ugedo L, Pineda J, Bleckmann S, Gass P, Kretz O, Mitchell JM, Schütz G, Maldonado R. Modulation of anxiety-like behavior and morphine dependence in CREB-deficient mice. Neuropsychopharmacology. 2004;29:1122–1133. doi: 10.1038/sj.npp.1300416. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Workman JL, Trainor BC, Finy MS, Nelson RJ. Inhibition of neuronal nitric oxide reduces anxiety-like responses to pair housing. Behav Brain Res. 2008;187:109–115. doi: 10.1016/j.bbr.2007.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Kim SS, Zhuo M. Molecular targets of anxiety: from membrane to nucleus. Neurochem Res. 2008;33:1925–1932. doi: 10.1007/s11064-008-9679-8. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, Zhu XJ, Wang B, Xu JS, Zhu DY. Neuronal nitric oxide synthase contributes to chronic stress-induced depression in mice. J Neurochem. 2007;103:1843–1854. doi: 10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- Zhu XJ, Hua Y, Jiang J, Zhou QG, Luo CX, Han X, Lu YM, Zhu DY. Neuronal nitric oxide synthase-derived nitric oxide inhibits neurogenesis in the adult dentate gyrus by down-regulating cyclic AMP response element binding protein phosphorylation. Neuroscience. 2006;141:827–836. doi: 10.1016/j.neuroscience.2006.04.032. [DOI] [PubMed] [Google Scholar]