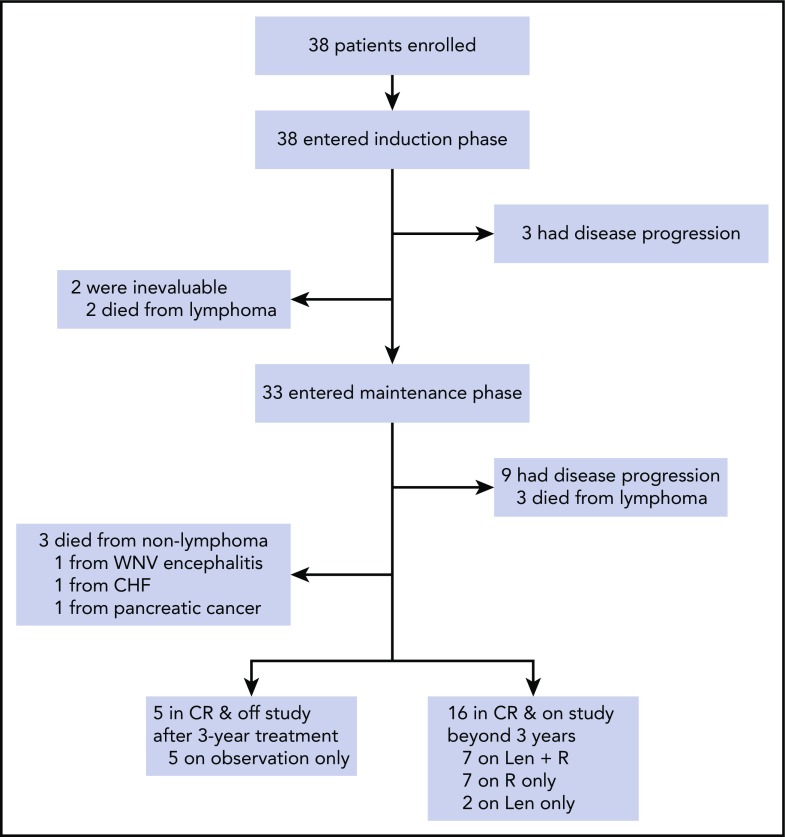
Figure 1.
Consort diagram of patient treatment and disposition. The induction treatment consisted of lenalidomide administered at 20 mg daily on days 1 to 21 of a 28-day cycle for 12 cycles and rituximab weekly for 4 weeks during cycle 1 and then every other cycle. Of the 38 patients enrolled, 33 completed induction and entered maintenance, whereas lenalidomide was reduced to 15 mg, and rituximab was continued every other cycle. Treatment was continuous until disease progression, unacceptable toxicity, or study withdrawal, with an option to stop therapy after 3 years. CHF, congestive heart failure; Len, lenalidomide, R, rituximab; WNV, West Nile virus.