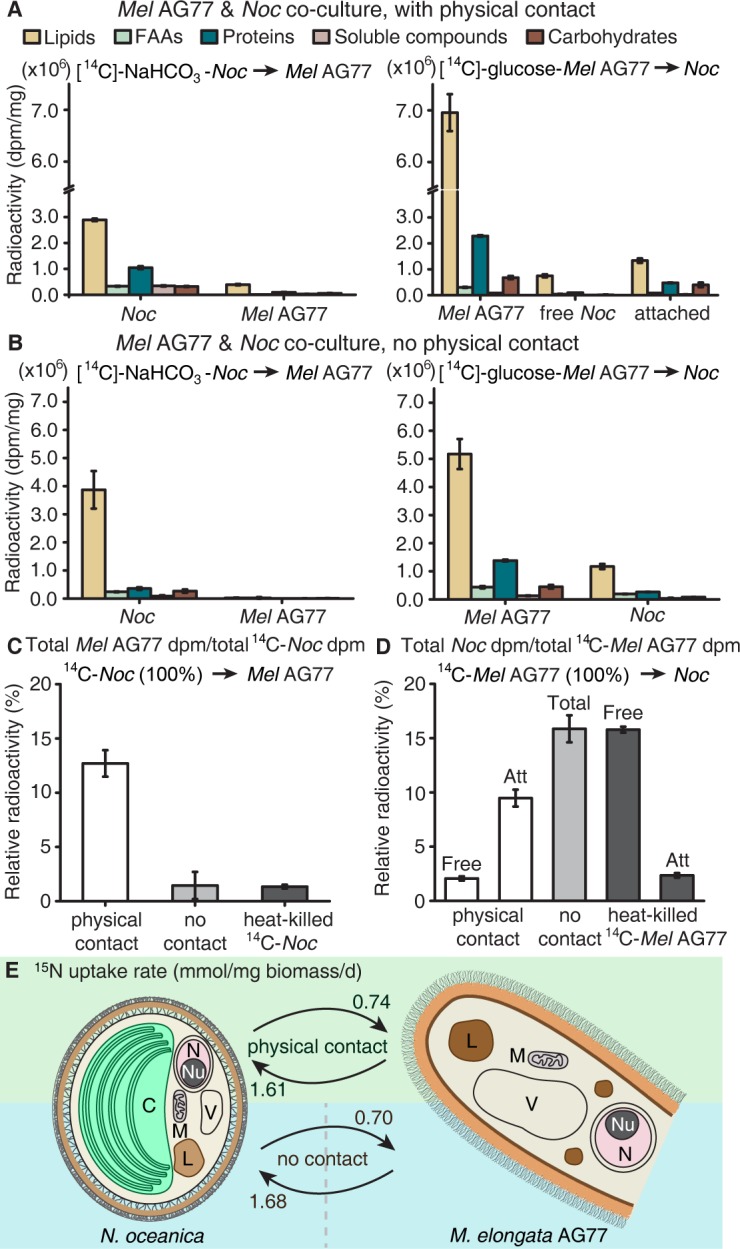
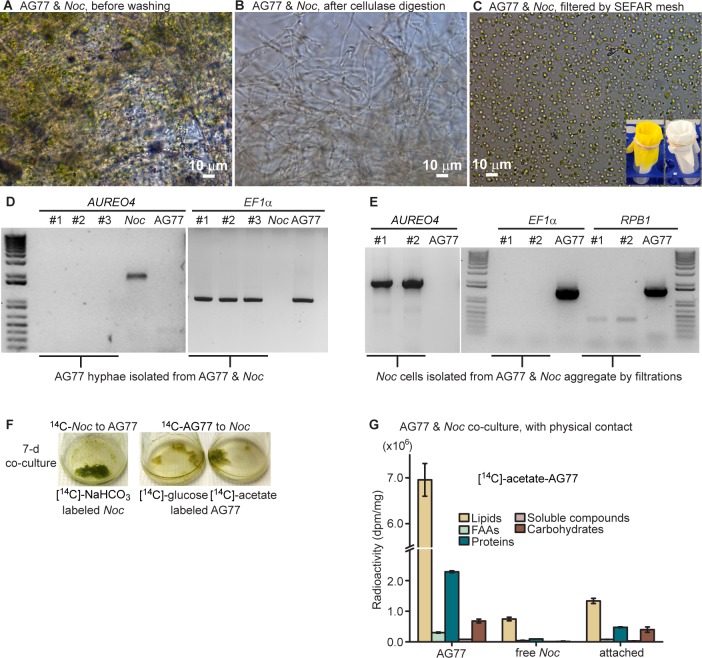
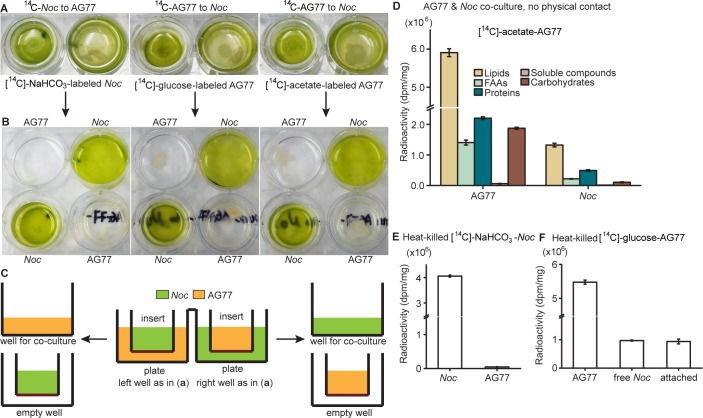
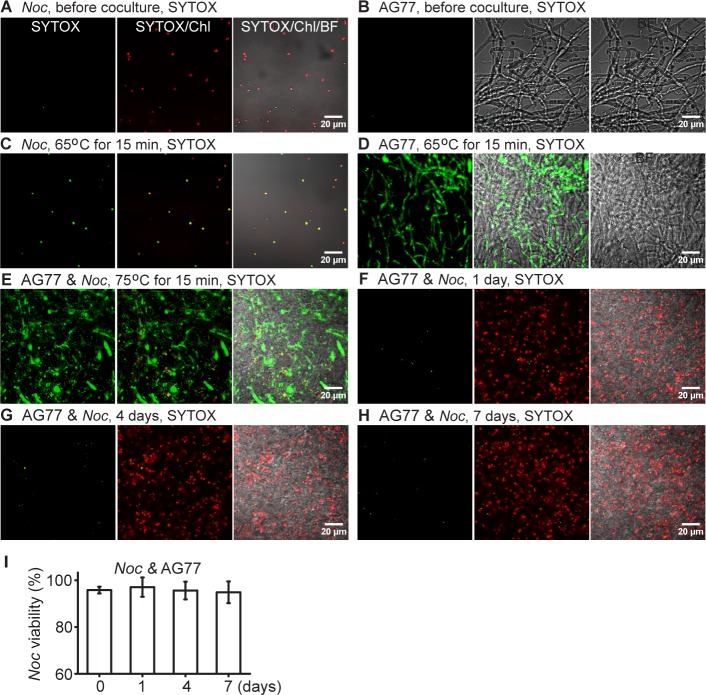
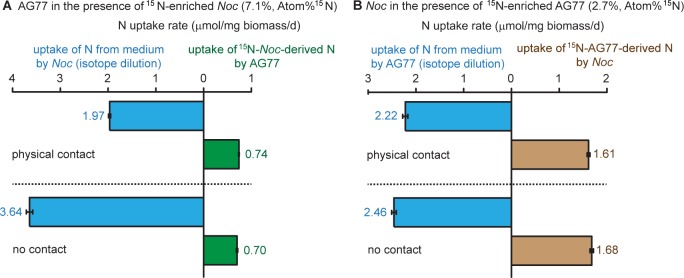
Figure 2. Carbon exchange between N.oceanica and M. elongata AG77.
(A) Carbon (C) transfer from [14C]-sodium bicarbonate (NaHCO3)-labeled N. oceanica (Noc) cells to M. elongata AG77 (Mel AG77, left panel) or from [14C]-glucose-labeled AG77 to Noc cells (right panel) after 7-d co-culture in flasks (with physical contact). Radioactivity of 14C-carbon was determined with a scintillation counter (dpm, radioactive disintegrations per minute) and then normalized to the dry weight of samples (dpm/mg biomass). Free Noc, unbound Noc cells in the supernatant; attached, Noc cells separated from AG77-Noc aggregates by algal cell wall digestion and mesh filtration; FAAs, free amino acids; soluble compounds, supernatant after acetone precipitation of proteins extracted by SDS buffer. Data are presented as the average of three biological replicates with standard deviation (Means ± SD, n = 3). (B) 14C-carbon transfer between Noc and AG77 without physical contact. Algae and fungi were incubated in cell-culture plates with filter-bottom inserts (pore size of 0.4 μm) which separate Noc cells and AG77 mycelium from each other but allow metabolite exchange during co-culture. Error bars indicate SD of three biological replicates (n = 3). (C and D) Relative abundance of 14C-carbon radioactivity in recipient cells compared to 14C-labeled donor cells after 7-d co-culture. (C) AG77 relative to [14C]-NaHCO3-Noc (100%). (D) Noc relative to [14C]-glucose-labeled AG77 (100%). Physical contact, living 14C-labeled cells added to unlabeled cells for co-cultivation in flasks; no contact, samples grown separately in plates with inserts; heat-killed 14C-cells, 14C-labeled Noc or AG77 killed by heat treatment at 65°C for 15 min before the addition to unlabeled cells in flasks. Free, unbound Noc cells in the supernatant; Att, Noc cells attached to AG77 (isolated by algal cell wall digestion and mesh filtration); Total, Noc cells grown separately from AG77 in plates and inserts. Error bars indicate SD of three biological replicates (n = 3). (E) Nitrogen (N) exchange between N. oceanica (Noc) and M. elongata AG77 examined by 15N-labeling experiments. [15N]-potassium nitrate-labeled Noc cells or [15N]-ammonium chloride-labeled AG77 were added to unlabeled AG77 or Noc cells, respectively, for 7-day co-culture in flasks (physical contact) or cell-culture plates with inserts (no physical contact). Algae and fungi were separated and weighed (dry biomass) after the co-culture, and their isotopic composition in Atom% 15N [15N/(15N+14N)100%] and N content (%N) were determined using an elemental analyzer interfaced to an Elementar Isoprime mass spectrometer following standard protocols. The N uptake rate of 15N-Noc-derived N (15N) by AG77 from and that of 15N-AG77-derived N by Noc cells (15N) were calculated based on the Atom% 15N, %N and biomass. C, chloroplast; N, nucleus; Nu, nucleolus; M, mitochondrion; V, vacuole; L, lipid droplet. Values are the average of three biological repeats.