Figure 3. N.oceanica benefits from co-culture with M. elongata.
(A–C) Viability assay of Noc cells and Noc co-cultured with AG77 under nitrogen (-N, (A) and carbon (-C, (B) deprivation. Dead Noc cells were indicated by SYTOX Green staining (green fluorescence). Red, Noc chlorophyll fluorescence. (C) Viability of nutrient-deprived Noc cells increased when co-cultured with two different M. elongata strains, AG77 and NVP64. Results are calculated from 1000 to 5000 cells of five biological repeats with ImageJ. Asterisks indicate significant differences compared to the Noc control as determined by Student’s t test (*p≤0.05, **p≤0.01; Means ± SD, n = 5). (D and E) Total organic C and dissolved N measurements in the buffer of 18-day fungal cultures of M. elongata strains AG77 and NVP64 compared to the f/2 medium control (f/2 con). Fungal cells were removed by 0.22-μm filters. Data are presented as the average of four biological replicates and asterisks indicate significant differences compared to the f/2 medium control as determined by Student’s t test. Means ± SD, n = 4. *p≤0.05, **p≤0.01.
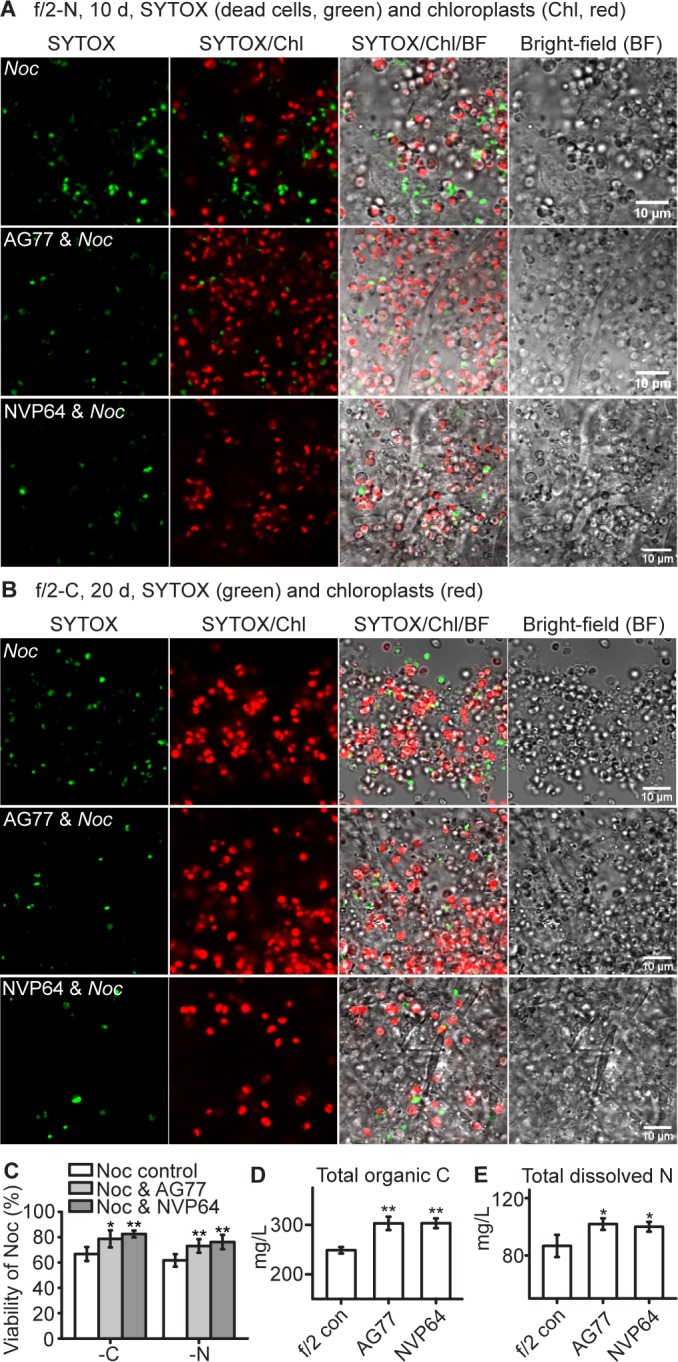
Figure 3—figure supplement 1. N. oceanica and M. elongata under stresses.
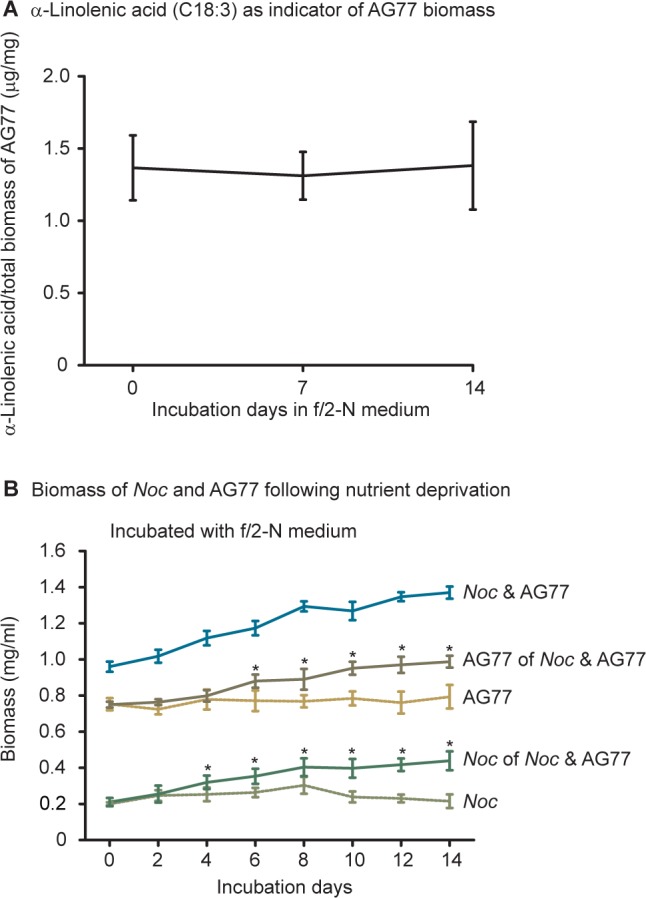
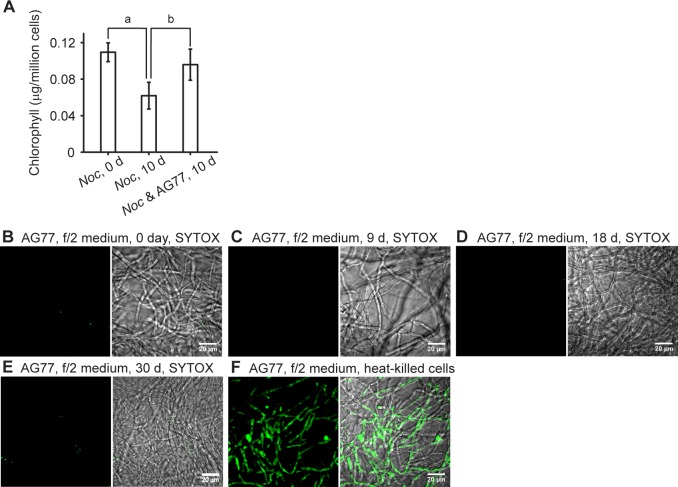
Figure 3—figure supplement 2. N.oceanica and M. elongata AG77 benefit from each other under nutrient starvation.