Table1.
Electronic effects of the TPMA family ligands.a
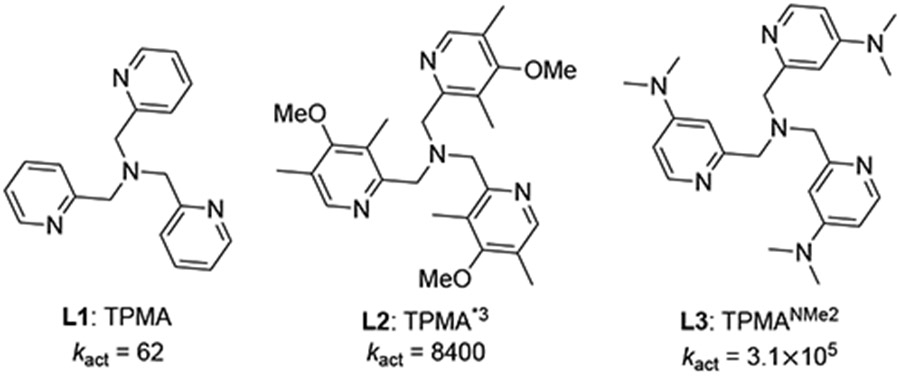 | ||||||
|---|---|---|---|---|---|---|
| ligand | ΔG‡exp | ΔG‡DFT | EHOMOb | Vbur%c | ΔEdist(TS)d | ΔEdist(BrCuIIL)e |
| TPMA | 15.0 | 16.6 | −7.66 | 39.2 | 2.1 | 4.0 |
| TPMA*3 | 12.0 | 14.6 | −7.41 | 40.3 | 2.0 | 3.6 |
| TPMANMe2 | 10.0 | 13.0 | −7.36 | 39.0 | 1.8 | 3.7 |
kact values are in M−1 s−1. All Gibbs free energies and distortion energies are in kcal/mol; EHOMO is in eV. All energies and EHOMO are computed in acetonitrile using the CPCM solvation model.
HOMO energy of [CuIL]+.
Percent buried volume of the ligand computed from the DFT-optimized geometry of [CuIL]+.41
Distortion energy of the CuL catalyst in the ISET transition state with respect to the ground state [CuIL]+.
Distortion energy of the CuL catalyst in the [BrCuIIL]+ complex with respect to the ground state [CuIL]+.
