Abstract
Glioblastoma (GBM) is a highly lethal brain tumor with poor responses to immunotherapies which have been successful in more immunogenic cancers with less immunosuppressive tumor microenvironments (TMEs). GBM’s TME is uniquely challenging to treat due to tumor cell extrinsic components that are native to the brain, as well as tumor intrinsic mechanisms which aid in immune evasion. Lowering the barrier of immunosuppression by targeting the genetically stable tumor stroma presents opportunities to treat the tumor in a way that circumvents the complications of targeting a constantly mutating tumor with tumor antigen directed therapies. Tumor associated monocytes, macrophages, and microglia (TAMs) are a stromal element of particular interest. Macrophages and monocytes compose the bulk of infiltrating immune cells and are considered to have pro-tumor and immunosuppressive effects. Targeting these cells or other stromal elements is expected to convert what is considered the “cold” TME of GBM to a more “hot” TME phenotype. This conversion could increase the effectiveness of what have become conventional frontline immunotherapies in GBM — creating opportunities for better treatment through combination therapy.
Keywords: glioblastoma, microenvironment, TAMs, brain, immunotherapy
Introduction
GBM is the most lethal and common primary brain tumor in adults. Despite an aggressive standard of care treatment regimen of surgical resection, radiochemotherapy, adjuvant chemotherapy, and tumor-treating fields prognosis remains poor with a 2-year survival rate of only 43% (1). While the implementation of immunotherapy has proven extremely successful in more immunogenic cancers, no survival benefit has been observed in GBM patients thus far. (2). The GBM TME shares components with these more treatable cancers but is also made unique by the brain tissue-resident cell types. In addition to these unique cellular components it is also insulated by the blood brain barrier (BBB), which contributes to the brain being widely considered a relatively immune privileged organ. Immune privileged organs have tightly regulated immune responses, which leads to a naturally more immunosuppressive environment.
In addition to tumor cell extrinsic components of the TME that lead to poor treatment response, there are several tumor-intrinsic properties that lead to poor immunogenicity and immunosuppression. GBM has recently been characterized into several subtypes based on the dominant aberrant transcriptional program. These are termed proneural (PN), mesenchymal (MES), and classical (CL) (3). There is a great degree of heterogeneity in these subtypes between patients, as well as within an individual tumor (4). In addition to the molecular subtypes based on global transcriptional programs, gliomas, including GBM, have also been stratified according to specific genomic aberrations: mutations in the Telomerase Reverse Transcriptase (TERT) promoter, alterations in the Isocitrate Dehydrogenase-1 (IDH) gene, and co-deletion of chromosome arms 1p and 19q (5). Regardless of GBM stratification method, mutations found in GBM are rarely homogenous and few of these mutations result in a surface protein modification that is unique to the brain tumor (6). As such, implementation of antigen-specific therapies is proving difficult and often results in immune escape (7).
In this review we discuss the components of the brain TME and how they may contribute to treatment response. We will also briefly review therapies that aim to directly target the TME to lower the barrier of immunosuppression in the hopes of making antigen specific therapies more effective.
Cellular Components of the Brain Tumor Microenvironment
The TME of GBM is unique in its cellular composition and accessibility to immune cells. The factors that make the TME unique are also what contribute to its highly immunosuppressive and “cold” TME phenotype. Unlike the consistently mutating tumor cells, the stroma of the TME is a genetically stable therapeutic target. Reducing the immunosuppression caused by these stromal cells has the potential to promote functional effector T cell infiltration and create new opportunities for treatment. Here we discuss how these non-immune (Figure 1) and immune (Figure 2) stromal elements contribute to immunosuppression and the “cold” TME phenotype.
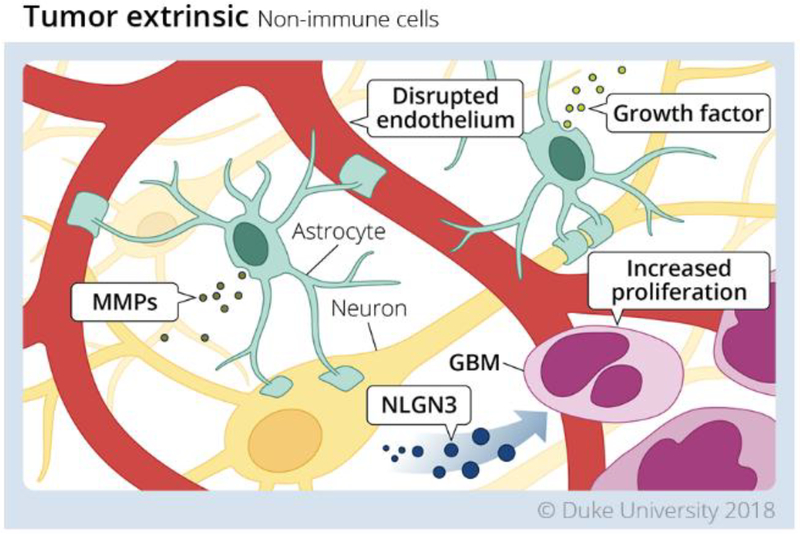
Figure 1. Tumor extrinsic mechanisms in Glioblastoma tumor microenvironment mediated by non-immune cells.
Non-immune cells contribute to the immunosuppressive environment of the GBM TME in the following ways. 1. Although it is disrupted the blood brain barrier remains selectively permeable to both effector cells, and therapeutics. 2. Astrocytes are a source of pro-tumor factors which support the growth and metastatic capability of tumor cells. 3. Neurons support tumor cell proliferation via secretion of NLGN3. Redrawn from an illustration by Megan Llewellyn, MSMI, CMI; copyright Duke University; with permission under a CC-BY 4.0 license.
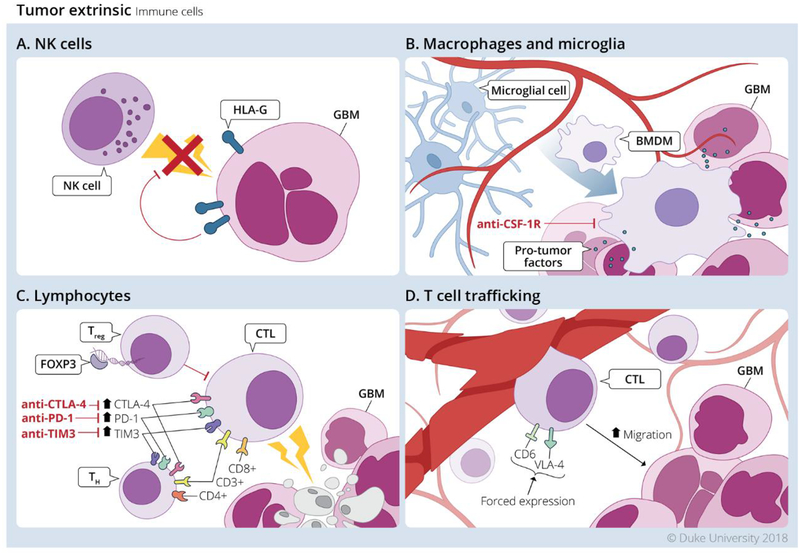
Figure 2. Tumor extrinsic mechanisms in Glioblastoma tumor microenvironment mediated by immune cells.
Infiltrating immune cells largely contribute to immunosuppression, or have their anti-tumor effector functions muted by exhaustion. A. NK cells have reduced cytotoxic capacity due to HLA-G expression on GBM cells. B. TAMs release pro-tumor factors which support tumor growth and suppress effector T cell function. C. TReg cells directly suppress CD8+ T cell cytotoxic capacity, and CD8+ and CD4+ T cells highly express exhaustion markers and are considered functionally exhausted. D. One potential method of increasing the immunogenicity of the GBM TME is by increasing trafficking to the tumor via forced expression of CD6 and VLA-4. Redrawn from an illustration by Megan Llewellyn, MSMI, CMI; copyright Duke University; with permission under a CC-BY 4.0 license.
Non-Immune Cellular Components
Vasculature
The BBB is functionally distinct organ structure of the brain, which has poor permeability to many frontline therapeutics and impedes the migration of some immune effectors under certain conditions. In the naïve state, the BBB provides a significant restriction to the permeability of many therapeutics especially large or hydrophilic molecules. However, the BBB loses its integrity in many pathologic situations including primary and malignant brain tumors and becomes increasingly permeable to therapies it would have otherwise blocked. Even within these pathologic situations there is a difference in permeability between gross tumor and infiltrated brain (8,9). A high degree of vascularity with atypical organization and reduced structural integrity is a common characteristic of GBM. This leakiness results in high interstitial fluid pressure, a great degree of hypoxia and necrosis, as well as edema (10). Vascularization of brain tumors, or angiogenesis, is considered unique due to the biological homology between vascular and neural networks (11). It has recently been suggested that neural stem cells and glioma stem cells can differentiate into endothelial cells within the glioma vasculature (12,13). This ability of glioma stem cells allows them to form glioma stem cell reservoirs in the perivascular niche (PVN), where they are insulated and can safely proliferate (14,15).
Glioma Stem Cells
Glioma Stem Cells (GSCs) are associated with the endothelial cells of the PVN (14,15). The number of GSCs associated with vessels in the TME strongly correlates with increasing tumor grade (16,17). A feedback loop exists between the endothelium and the GSCs as the endothelium releases factors that drive tumor sphere formation and GSCs release factors that accelerate angiogenesis (16). Nitric Oxide (NO) is one such endothelium derived factor that reinforces stem-cell like characteristics in GSCs (18). GSCs have been shown to additionally recruit monocytes to the TME and polarize them to a pro-tumor phenotype via secretion of CCL2 and CSF-1 (19). GSCs also directly inhibit T cell activation, proliferation, and induce T cell apoptosis (20). Lastly, it has been suggested that GSCs induce functionally active TReg cells (20). These effects combined result in GSCs mediating a great degree of immunosuppression while remaining difficult to target.
Astrocytes
Astrocytes provide structural support in the brain by maintaining homeostasis. They are typically localized to the PVN and play an important role in maintenance of the BBB (21). Astrocytes are thought to have pro-tumor functions via secretion of neurotrophic factors which support proliferation of glioma cells (15). In the naïve brain activated astrocytes supply growth factors and cytokines to enable the repair of brain tissue during different forms of injury. This process is referred to as reactive gliosis, which is one mechanism of wound healing in the brain (22). In the TME these growth factors have been shown to support tumor growth and mediate resistance to therapy (23). In addition to supplying growth factors, they also secrete metalloproteinases which create a favorable environment for tumor invasion (24).
Neurons
Neurons are a brain-specific cell type, like astrocytes, that are thought to contribute to the creation and outgrowth of tumors. Neurons provide mitogenic signals within the brain to drive neural stem cell growth (25). Recent studies show that neuron derived neuroligin-3 (NLGN3) increases proliferation of tumor cells via tumor intrinsic PI3K signaling. It was also shown that in human GBM NLGN3 expression inversely correlates with survival (26). In breast cancer brain metastases, it has also been shown that increased neurotransmitters released by neurons serve as an oncometabolite (27). Whether this process occurs in GBM as well remains to be determined, but it serves as an example where neuron-derived products serve pro-tumor roles in the brain TME.
Immune Cellular Components
Tumor-infiltrating lymphocytes (TILs)
TILs have the potential to exert both pro- and anti-tumor functions in the TME. T cells are the primary lymphoid component of the TME but compose less than 0.25% of cells isolated from human GBM biopsies (28). CD8+ cytotoxic T Lymphocytes (CTLs) are considered critical for tumor clearance, but account for less than a quarter of the already sparse TIL population of the TME (28). Functional characterization of the CTLs found in the TME has shown that these cells have impaired effector functions and an exhausted phenotype, rendering them ineffective in their role as cytotoxic lymphocytes (29). Similarly, CD4+ T helper cells, which typically have anti-tumor functions, may correlate with poor survival outcomes (30). This is likely explained by a large percentage of the CD4+ TIL population being TReg cells, and the remainder being functionally exhausted (31). FOXP3+ CD25+ TReg cells, a CD4 subset, are functionally immunosuppressive. Efforts aimed at depleting these pro-tumor lymphocytes have had modest effects at increasing CTL function in GBM (31).
Tumor-associated macrophages (TAMs)
Bone marrow-derived macrophages/monocytes (BMDMs) are the primary immune cell in GBM and compose up to 30% of the tumor mass (32). There are two distinct macrophage populations in the GBM TME, BMDMs and Microglia (33,34). Collectively these are referred to as TAMs. Unlike BMDMs, Microglia develop from yolk sac progenitor cells, and are not replenished post-natally by hematopoiesis (35). During tumor progression, monocytes and macrophages can extravasate into the TME through the compromised BBB (36). Conventionally, macrophages have been thought to exist in either the inflammatory (M1) or wound healing (M2) phenotype. Recent works suggests that this is a gross over-simplification due to the staggering functional diversity and plasticity of TAMs across tumor types (37). In the GBM TME TAMs have a distinct pro-tumor role and their accumulation correlates with tumor grade (34). Functionally, TAMs in the GBM TME, are only producing low levels inflammatory cytokines and lack the ability to aid in T cell responses via co-stimulation (38). Additionally, they have been found to be great contributors to the immunosuppressive TME via release of soluble factors that dampen the immune response (39).
Natural Killer Cells
Natural Killer cells are innate lymphoid cells that identify and kill tumor cells by sensing danger or damage signals (40). NK cells are found in GBM and have been shown to be effective at inducing lysis in GSCs (41). Unfortunately, GBM is known to express HLA-G which acts as an inhibitory ligand for activated NK cells (42). This likely aids in their evasion from NK cell-mediated cell killing. Macrophages have been shown to mediate NK cell activation, but lose this priming ability when macrophages adopt a pro-tumor phenotype (43). A definitive link between TAM polarization and NK cell activation has not yet been proven in GBM however. Like TAMs, NK cells are a component of the GBM TME that possess the ability to lyse tumor cells in an antigen-independent manner but are rendered functionally suppressed.
Tumor Cell-Intrinsic Mutations Contribute to “Cold Tumor” Phenotype
The interplay between the cancerous cells and the surrounding stroma is critical in developing the TME present with gliomas. The stroma response to tumor extrinsic factors such as constant cycles of hypoxia, acidosis, necrosis, angiogenesis and granulation (44,45). These processes were originally perceived to be the responsible for the immunosuppressive nature of the TME. This constant state of “chronic inflammation” was what originally granted tumors the term “the wound that never heals” (46). However, this original belief fails to explain how tumors of conventional histology and location can have disparate TME between patients (47). Factors such as age, HLA-type, and genetics may explain some differences between patients, however, these factors do not account for the recent observations where different tumor lesions located within the same organ in a patient can have different TME characteristics (48). Therefore, while tumor-extrinsic factors definitely contribute to the formation of the immunosuppressive TME, tumor-intrinsic factors in the form of tumor genetic or epigenetic changes have recently been demonstrated to play a critical role in shaping this milieu (Figure 3).
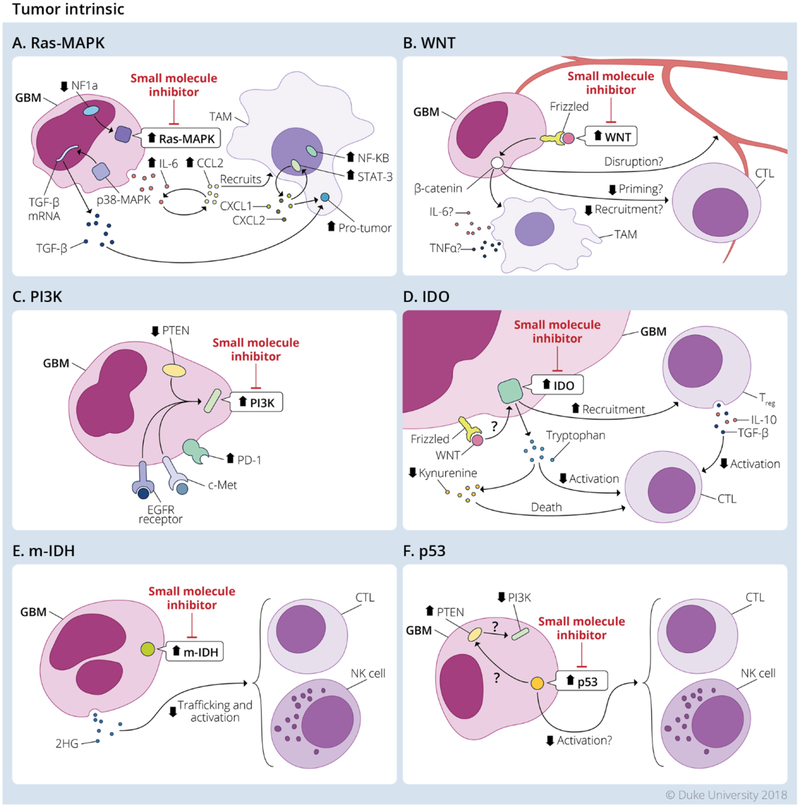
Figure 3. Tumor intrinsic mechanisms in Glioblastoma tumor microenvironment.
Tumor intrinsic mechanisms present in GBM shown to modulate the TME and contribute to immunosuppression. The main pathways altered in GBM are the: A. Ras-Mitogen Activated Protein Kinase (Ras-MAPK); B. WNT/β-catenin (WNT); C. Phosphoinositide 3-kinase (PI3K); D. indoleamine 2 3-dioxygenase (IDO); E. Isocitrate Dehydrogenase-1 (m-IDH); and F. p53. Small molecule inhibitors targeting these pathways are currently in development for clinical application. Redrawn from an illustration by Megan Llewellyn, MSMI, CMI; copyright Duke University; with permission under a CC-BY 4.0 license.
The results obtained from the clinical trials employing checkpoint blockade strategies have highlighted the relevance of the role of tumor intrinsic factors in the establishment of the TME, and response to therapy. This field has divided tumors into “cold tumors” versus “hot tumors”, where “cold tumors” are characterized by the lack of T cell infiltrate within the TME, while “hot tumors” are infiltrated with mostly CD8+ T cells. many of which appear locally activated yet are extrinsically suppressed (49). It is currently believed that “cold tumors” are less responsive to immunotherapies while “hot tumors” are primed to respond. The majority of GBM samples display a “cold tumor” phenotype with few CD8+ TIL.
GBM is a high grade tumor that arises from astrocytic origin and it is largely confined to the Central Nervous System (CNS). Extrinsic factors that directly impact the TME and T cell penetration have been previously characterized. As mentioned above, the presence of a BBB, neurons, microglia and surrounding astrocytes are critical in shaping the TME. The direct interaction of these components with the tumor appears to be regulated by tumor intrinsic mutations. Tumor intrinsic factors resulting from the Phosphoinositide 3-kinase (PI3K), Ras-Mitogen Activated Protein Kinase (Ras-MAPK), WNT/β-catenin, p53, and IDH pathways have been shown to modulate the TME and facilitate the emergence of “cold tumors” (50). Coincidentally, despite GBM containing an average of 40 mutations per tumor, whole genome sequencing of GBM tumor samples have revealed that the main pathways altered in GBM are the: PI3K, Ras-MAPK, p53, and IDH (3,51). The WNT/β-catenin pathway has not been shown to be commonly mutated yet the pathway appears to be active within a proportion of GBM tumors (6). While most of the studies of these pathways in GBM are focused on how these regulate proliferation, survival and invasion, more research is required to determine their role in shaping immune resistance.
PI3K pathway
PI3K is a major pathway active in GBM, which can be activated in multiple ways. Signaling from the Epidermal Growth Factor Receptor (EGFR) (52), and c-mesenchymal-epithelial transition receptor (c-met) are common pathways responsible for PI3K activation (53). Loss-of-function mutations of the tumor suppression Phosphatase and tensin homolog (PTEN), which results in constitutively active PI3K signaling, is commonly seen in GBM (54). Active PI3 Kinase in GBM has been shown to result in the expression of the immune checkpoint ligand PD-L1 (55). The interaction of PD-L1 with the receptor PD-1 is one of the main pathways to suppress T cell responses within the tumor context.
Ras-MAPK
The Ras-MAPK pathway is another major pathway in GBM downstream of the EGFR (52) and c-met receptors as well (53). Many GBM samples harbor loss-of-function mutation on the NF1 gene, which results in further Ras activation (56). Active Ras-MAPK pathway has also shown to contribute to modulation of the TME through the induction of IL-6 mRNA (57). IL-6 is a pleiotropic cytokine which is known to induce CCL2 expression. CCL-2 is an abundant chemokine within GBM tumors and has been shown to mediate recruitment of monocytes into tumors. IL-6 also leads to the activation of NF-κB and STAT-3 in infiltrating monocytes as well as GSCs, which are known to express the IL-6 Receptor (58). Active STAT-3 promotes the recruitment of monocytes via CXCL1, CXCL2 expression, and the induction of a suppressive TME by inducing macrophages to the pro-tumor phenotype (58). In addition to the induction of IL-6 Ras-MAPK signaling process includes the activation of p38 MAP kinase (59), which plays a critical role in the induction of TGF-β mRNA (60). TGF-β is a central pleiotropic cytokine in shaping the immunosuppressive TME, inducing the pro-tumor TAM phenotype, impairing DC migration and cytokine sections, inhibiting T cell responses, and limits the infiltration of leukocytes into the tumor (61). While it is not well known if GBM mutations have an intrinsic effect in regulating TGF-β expression, post-transcriptional regulation is critically dependent on the Ras-MAPK-p38 pathway.
WNT/β-catenin
The WNT-β-catenin pathway has recently been explored in more detail in GBM samples. While this pathway does not appear to be frequently mutated in GBM, there is significant epigenetic regulation which leads to elevated levels of WNT proteins such as Wnt5A (6). WNT proteins bind to Frizzled receptors and signal through the β-catenin protein, which enters the nucleus and binds transcription factors to activate gene transcription (62). The WNT/β-catenin pathway has recently been shown to play a pivotal role in the melanoma TME, leading to the establishment of “cold tumors” by its effect on limiting DC infiltration and blunting both T cell priming and effector T cell recruitment (63). The relevance of this pathway in the TME of GBM is currently being investigated, but one of the well-known effects of WNT/β-catenin pathway is its role in the maintenance of the BBB integrity (64). In addition, it is known to further promote the secretion of IL-6 and TNFα in infiltrating TAMs. As this pathway is further elucidated it remains critical to determine if the observations seen in melanoma translates into GBM tumors.
p53
The well-known tumor suppressor p53 is largely mutated in GBM tumors (3). The loss-of-function mutation is believed to occur early during tumorigenesis. Loss-of-function mutations in the p53 gene result in increased proliferation, reduced cell death, and genetic instability (65). Recent studies have also shown that the loss p53 function, reduces the induction of inflammatory cytokines capable of alerting the immune system and activating NK cells (66). Re-introduction of p53 in GBM tumors leads to the induction of TNFα, and resulted in increased TIL populations (67). It is expected that p53 could sense DNA damage in tumors and induce PTEN expression in those tumors which PTEN is functional, and reduce PI3K activity as well (68), resulting in reduced immunosuppression. More studies are required to elucidate the detailed functional role of p53 in GBM in regulating the “cold tumor” phenotype.
IDH
Mutations can occur in either IDH1 or IDH2 and since these genes are very similar they will collectively be referred to as IDH throughout. IDH is a ubiquitous enzyme responsible for catalyzing the conversion of Isocitrate into 2-oxoglutarate as part of the tricarboxylic acid cycle (TCA) cycle (69). IDH has been shown to be mutated in ~10% of primary GBM, but in as many as 90% secondary GBMs (70). It has recently been demonstrated that the IDH mutation IDH R132H, results in the production of the oncometabolite (D) 2-Hydroxygluterate (2HG), which directly modulates the TME reducing chemo-attractants responsible for the recruitment of TILs and limiting the function of NK cells (71–73). More studies are in necessary to address other potential mechanisms by which IDH R132H and its oncometabolite, 2HG, modulate the TME in GBM.
IDO
One pathway which etiology still needs to be defined in GBM is the Indolamine 2,3-dioxygenase (IDO). This pathway has recently been demonstrated to be active in GBM tumors as well as in surrounding stroma (74). IDO is generally activated upon IFN-γ signaling or B7 signaling in dendritic cells. However, it remains unknown how this is activated in GBM tumor cells (75). Recent studies highlight the possibility of WNT/ β-catenin signaling in regulating IDO expression (76). Its expression and enzymatic activity leads to reduce tryptophan levels, an essential amino acid, and increases the synthesis of toxic kynurenine metabolites (77). Decreased levels of tryptophan reduce T cell activity, and increased kynurenine metabolites lead to T cell death (77). IDO is also responsible for TReg cells recruitment and activation within the GBM tumors (78). TReg cells are then capable of shaping the surrounding TME by secreting IL-10, and TGF-β, therefore leading to M2 polarization and immunosuppression.
Mutations intrinsic to the tumor, in the previously mentioned pathways, have direct consequences on shaping the TME, and serve to promote the “cold” tumor phenotype; however, the resultant environment is also caused by the interplay between the tumor itself and surrounding stroma. Production of cytokines like IL-6 and stabilization of cytokines such as TGF-β signal the surrounding stroma to activated molecules such as STAT-3. This results in the release of chemokines responsible for monocyte and macrophage recruitment. These cells will in turn interact with the TGF-β, as well as respond to the tumor extrinsic factors such as hypoxia, acidosis, and necrosis to establish the TME characteristic of GBM.
Immune Interventions
Immunotherapeutic interventions aimed at lowering the barrier of immunosuppression and converting the TME from a “cold” to a “hot” phenotype will be instrumental for improving success of frontline immunotherapies in GBM. Having large amounts of functional CTLs in the TME is known to correlate with treatment outcomes and survival across solid tumors, and CTL function is predicated on sufficiently low immunosuppressive factors in the TME. Here we discuss how different immune interventions may serve to reduce these immunosuppressive factors.
TAM-directed strategies
TAMs serve as a genetically stable target for therapeutic intervention. While they take on a pro-tumor phenotype in the GBM TME, efforts to re-educate TAMs to a more inflammatory state has becoming a topic of great interest (79). Microglia are dependent on Colony Stimulating Factor-1 (CSF-1) and pharmacologic interventions aimed at inhibiting the CSF-1 receptor (CSF-1R) have shown the ability to either deplete or re-program TAMs in some preclinical murine models (80–82). While programming TAMs to an anti-tumor phenotype is expected to be more effective than depletion, the plasticity of TAMs likely leads to a less durable anti-tumor phenotype. Finding ways to irreversibly polarize TAMs to an anti-tumor phenotype without reducing their migration into the TME could greatly reduce the degree of immunosuppression, and potentially open the door to antigen specific therapies becoming more effective.
Small Molecule Inhibitors
Small molecule inhibitors targeting the PI3K, Ras-MAPK, signaling pathways and the IDH and IDO enzymatic activity have recently been developed and are under current evaluation in combination with active immunotherapy or checkpoint blockade. The original purpose of these agents were to directly limit the pro-tumorigenic activity of these pathways, however, most of these inhibitors failed when utilized as a single agent due to the large degree of tumor genetic heterogeneity present within and across GBM patients (83,84). Nonetheless, despite their failure to mediate antitumor efficacy as single agents, the combination of these inhibitors is expected to alter the TME in ways that might allow the immune system to infiltrate mediate tumor rejection, when combined with other immune modulatory agents such as vaccines, adoptive therapy or checkpoint blockade.
Immune Checkpoint Blockade Therapy
Immune Checkpoint Blockade Therapy (ICBT) involves the targeting of mechanisms that exist to maintain self-tolerance via inhibition of T cell activation. In the GBM TME exhausted TILs are known to have elevated expression of Cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), programmed cell death receptor-1 (PD-1), and T-cell inhibitory receptor 3 (TIM-3) (85–87). Monoclonal antibodies (mAbs) blocking these receptors have shown promise and even gained approval in a variety of cancers (88). Unfortunately, use of these antibodies as monotherapies or combination therapy failed in a Phase III clinical trial for use in GBM (89). Despite the inability of ICB to restore tumoricidal function of T cells in GBM, it remains promising as an addition to other TME-directed treatments as a means of further reducing immunosuppression.
Improving T cell Trafficking
Successful immunotherapy requires the presence of functional T cells within tumors, but as we previously mentioned the immunosuppressive nature of the GBM TME limits T cell infiltration, and TILs capable of infiltrating the tumor are rendered dysfunctional (29). Thus, methods which improve T cell infiltration like in situ cytokine infusion aimed to increase migration to the TME, or T cell modification which enhances migratory capacity into the TME, are highly desirable. While several strategies to modulate the GBM TME via cytokine infusion have shown to increase T cell infiltration, function and efficacy (90–92), licensing T cells to migrate into the TME has proven challenging. Previous studies focused on enhancing the expression of the adhesion molecule Very Late Antigen-4 (VLA-4) (93), which resulted in modest enhancements in infiltration and antitumor efficacy. A recent study identified a previously unrecognized role for the adhesion molecule CD6 (94), as a modulatory target capable of selectively increasing T cell infiltration into the GBM TME. Therefore, GBM TME modulation through direct infusion of immunomodulatory cytokines or forced expression of the adhesion molecules VLA-4 or CD6 on T cells could be beneficial for improving the selective infiltration of functional T cells within brain TME. The application of these strategies in combination with the above-mentioned strategies aimed at improving T cell function within the GBM TME could synergize in overcoming the immunosuppressive barrier present within GBM and mediate tumor eradication.
Conclusions
GBM’s TME is extremely immunosuppressive due to tumor intrinsic and extrinsic components. This leads to unique challenges in treating this cancer. Many therapies that have succeeded in more immunogenic cancers have failed as a result of this immunosuppression. Due to the great degree of heterogeneity and the adaptive nature of tumors, antigen-specific therapies will likely need to be supplemented by therapies which aim to directly reduce immunosuppression via targeting the genetically stable stroma. Considering the abundance of TAMs, their genetic stability and their paramount role in the maintenance of the immunosuppressive TME within GBM, we expect that TAM-directed immunotherapeutic strategies would greatly reduce the degree of immunosuppression present in GBM. TAM-directed immunotherapeutic strategies could promote T cell effector function and trafficking thereby shifting the GBM TME from “cold” to a “hot” phenotype. Furthermore, combination therapy including TAM-directed therapies and checkpoint inhibitors may synergize in enhancing the antitumor effect of TILs. In order to determine the effect of these TAM-directed immunotherapeutic strategies, clinical trials should focus on evaluating brain tumor penetration by TILs, on-target effects on TAMs and changes in immunosuppressive markers in the TME. GBM offers the possibility of evaluating these agents in the neo-adjuvant setting, which could then allow for tumor resection and careful examination of the GBM TME. Discovering how different subtypes of GBM respond best to different TAM-directed therapies could make possible the use of individualized antigen specific treatments, which have a great degree of neo-epitope coverage.
Funding:
This work was supported by grants from the National Institutes of Health: R01-NS099463 (Sampson), P50-CA190991 (Sampson), R01-CA177476 (Sampson), U01-NS090284 (Sampson), and R01-NS085412 (Sampson). The funders had no role in the preparation of the manuscript or decision to publish. The funders had no role in the preparation of the manuscript or decision to publish.
Footnotes
Conflicts of Interest: J.H.S. has an equity interest in Annias Immunotherapeutics, which has licensed intellectual property from Duke related to the use of the pepCMV vaccine in the treatment of glioblastoma multiforme. J.H.S. has an equity interest in Istari Oncology, which has licensed intellectual property from Duke related to the use of poliovirus and D2C7 in the treatment of glioblastoma. J.H.S. is an inventor on patents related to PEP-CMV DC vaccine with tetanus, as well as poliovirus vaccine and D2C7 in the treatment of glioblastoma.
References
- 1.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017;318(23):2306–16 doi 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampson JH, Maus MV, June CH. Immunotherapy for Brain Tumors. Journal of Clinical Oncology 2017;35(21):2450–6 doi 10.1200/jco.2017.72.8089. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008;455(7216):1061–8 doi 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sottoriva A, Spiteri I, Piccirillo SGM, Touloumis A, Collins VP, Marioni JC, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proceedings of the National Academy of Sciences of the United States of America 2013;110(10):4009–14 doi 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. The New England journal of medicine 2015;372(26):2499–508 doi 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B, Li T, Pignon J-C, Wang B, Wang J, Shukla S, et al. Landscape of tumor-infiltrating T cell repertoire of human cancers. Nature genetics 2016;48(7):725–32 doi 10.1038/ng.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. New England Journal of Medicine 2016;375(26):2561–9 doi 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodworth GF, Dunn GP, Nance EA, Hanes J, Brem H. Emerging Insights into Barriers to Effective Brain Tumor Therapeutics. Frontiers in Oncology 2014;4:126 doi 10.3389/fonc.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. Journal of Inherited Metabolic Disease 2013;36(3):437–49 doi 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 10.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience 2006;7:41 doi 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 11.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nature Reviews Neuroscience 2007;8:610 doi 10.1038/nrn217510.1038/nrn2175https://www.nature.com/articles/nrn2175#supplementary-informationhttps://www.nature.com/articles/nrn2175#supplementary-information . [DOI] [PubMed] [Google Scholar]
- 12.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010;468:824 doi 10.1038/nature0955710.1038/nature09557https://www.nature.com/articles/nature09557#supplementary-informationhttps://www.nature.com/articles/nature09557#supplementary-information . [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010;468:829 doi 10.1038/nature0962410.1038/nature09624https://www.nature.com/articles/nature09624#supplementary-informationhttps://www.nature.com/articles/nature09624#supplementary-information . [DOI] [PubMed] [Google Scholar]
- 14.Charles N, Holland EC. The perivascular niche microenvironment in brain tumor progression. Cell Cycle 2010;9(15):3012–21 doi 10.4161/cc.9.15.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell stem cell 2014;14(3):357–69 doi 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007;11(1):69–82 doi 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Bradshaw A, Wickremsekera A, Tan ST, Peng L, Davis PF, Itinteang T. Cancer Stem Cell Hierarchy in Glioblastoma Multiforme. Frontiers in Surgery 2016;3:21 doi 10.3389/fsurg.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charles N, Ozawa T, Squatrito M, Bleau A-M, Brennan CW, Hambardzumyan D, et al. Perivascular Nitric Oxide Activates Notch Signaling and Promotes Stem-like Character in PDGF-induced Glioma Cells. Cell stem cell 2010;6(2):10.1016/j.stem.2010.01.001 doi 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu A, Wei J, Kong L-Y, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncology 2010;12(11):1113–25 doi 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J, Barr J, Kong L-Y, Wang Y, Wu A, Sharma AK, et al. Glioblastoma Cancer-Initiating Cells Inhibit T-Cell Proliferation and Effector Responses by the Signal Transducers and Activators of Transcription 3 Pathway. Molecular Cancer Therapeutics 2010;9(1):67–78 doi 10.1158/1535-7163.Mct-09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell and Tissue Research 2009;335(1):75–96 doi 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- 22.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nature reviews Neuroscience 2015;16(5):249–63 doi 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF1R inhibition in gliomas. Science (New York, NY) 2016;352(6288):aad3018-aad doi 10.1126/science.aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le DM, Besson A, Fogg DK, Choi K-S, Waisman DM, Goodyer CG, et al. Exploitation of Astrocytes by Glioma Cells to Facilitate Invasiveness: A Mechanism Involving Matrix Metalloproteinase-2 and the Urokinase-Type Plasminogen Activator–Plasmin Cascade. The Journal of Neuroscience 2003;23(10):4034–43 doi 10.1523/jneurosci.23-10-04034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Sage JC, Miller MR, Verhaak RGW, Hippenmeyer S, Vogel H, et al. Mosaic Analysis with Double Markers (MADM) Reveals Tumor Cell-of-Origin in Glioma. Cell 2011;146(2):209–21 doi 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015;161(4):803–16 doi 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM, et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proceedings of the National Academy of Sciences of the United States of America 2014;111(3):984–9 doi 10.1073/pnas.1322098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S, Ma E, Wang X, Yu C, Dong T, Zhan W, et al. Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncology Letters 2016;12(4):2924–9 doi 10.3892/ol.2016.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-Cell Dysfunction in Glioblastoma: Applying a New Framework. Clinical Cancer Research 2018. doi 10.1158/1078-0432.Ccr-18-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, et al. Tumour-infiltrating CD4+ and CD8+ lymphocytes as predictors of clinical outcome in glioma. British Journal Of Cancer 2014;110:2560 doi 10.1038/bjc.2014.16210.1038/bjc.2014.162https://www.nature.com/articles/bjc2014162#supplementary-informationhttps://www.nature.com/articles/bjc2014162#supplementary-information . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, et al. Systemic Anti-CD25 Monoclonal Antibody Administration Safely Enhances Immunity in Murine Glioma without Eliminating Regulatory T Cells. Clinical Cancer Research 2006;12(14):4294–305 doi 10.1158/1078-0432.Ccr-06-0053. [DOI] [PubMed] [Google Scholar]
- 32.GM B, SB W, KG W. Microglia in brain tumors. Glia 2002;40(2):252–9 doi doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- 33.Bowman RL, Joyce JA. Therapeutic targeting of tumor-associated macrophages and microglia in glioblastoma. Immunotherapy 2014;6(6):663–6 doi 10.2217/imt.14.48. [DOI] [PubMed] [Google Scholar]
- 34.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nature neuroscience 2016;19(1):20–7 doi 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk sac-derived erythro-myeloid progenitors. Nature 2015;518(7540):547–51 doi 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews Immunology 2011;11(11):762–74 doi 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nature Immunology 2015;17:34 doi 10.1038/ni.3324. [DOI] [PubMed] [Google Scholar]
- 38.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology 2006;8(3):261–79 doi 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wurdinger T, Deumelandt K, van der Vliet HJ, Wesseling P, de Gruijl TD. Mechanisms of intimate and long-distance cross-talk between glioma and myeloid cells: how to break a vicious cycle. Biochim Biophys Acta 2014;1846(2):560–75 doi 10.1016/j.bbcan.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Lowry LE, Zehring WA. Potentiation of Natural Killer Cells for Cancer Immunotherapy: A Review of Literature. Frontiers in Immunology 2017;8:1061 doi 10.3389/fimmu.2017.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, et al. NK Cells Recognize and Kill Human Glioblastoma Cells with Stem Cell-Like Properties. The Journal of Immunology 2009;182(6):3530–9 doi 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 42.Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, et al. A Functional Role of HLA-G Expression in Human Gliomas: An Alternative Strategy of Immune Escape. The Journal of Immunology 2002;168(9):4772–80 doi 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 43.Bellora F, Castriconi R, Dondero A, Reggiardo G, Moretta L, Mantovani A, et al. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proceedings of the National Academy of Sciences of the United States of America 2010;107(50):21659–64 doi 10.1073/pnas.1007654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001;1(1):46–54 doi 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol 2017;43:74–89 doi 10.1016/j.semcancer.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res 2015;3(1):1–11 doi 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016;165(1):35–44 doi 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacquelot N, Roberti MP, Enot DP, Rusakiewicz S, Ternes N, Jegou S, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 2017;8(1):592 doi 10.1038/s41467-017-00608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv Exp Med Biol 2017;1036:19–31 doi 10.1007/978-3-319-67577-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nature Reviews Cancer 2018;18:139 doi 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360(8):765–73 doi 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 2017;9(5) doi 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abounader R, Ranganathan S, Kim BY, Nichols C, Laterra J. Signaling pathways in the induction of c-met receptor expression by its ligand scatter factor/hepatocyte growth factor in human glioblastoma. J Neurochem 2001;76(5):1497–508. [DOI] [PubMed] [Google Scholar]
- 54.Koul D PTEN signaling pathways in glioblastoma. Cancer Biol Ther 2008;7(9):1321–5. [DOI] [PubMed] [Google Scholar]
- 55.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007;13(1):84–8 doi 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 56.McGillicuddy LT, Fromm JA, Hollstein PE, Kubek S, Beroukhim R, De Raedt T, et al. Proteasomal and genetic inactivation of the NF1 tumor suppressor in gliomagenesis. Cancer Cell 2009;16(1):44–54 doi 10.1016/j.ccr.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao W, Liu M, Kirkwood KL. p38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. J Biol Chem 2008;283(4):1778–85 doi 10.1074/jbc.M707573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeung YT, McDonald KL, Grewal T, Munoz L. Interleukins in glioblastoma pathophysiology: implications for therapy. Br J Pharmacol 2013;168(3):591–606 doi 10.1111/bph.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munoz L, Yeung YT, Grewal T. Oncogenic Ras modulates p38 MAPK-mediated inflammatory cytokine production in glioblastoma cells. Cancer Biol Ther 2016;17(4):355–63 doi 10.1080/15384047.2016.1139249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao YQ, Freire-de-Lima CG, Schiemann WP, Bratton DL, Vandivier RW, Henson PM. Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J Immunol 2008;181(5):3575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.David CJ, Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol 2018;19(7):419–35 doi 10.1038/s41580-018-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnes M, Casas Tinto S. Aberrant Wnt signaling: a special focus in CNS diseases. J Neurogenet 2017;31(4):216–22 doi 10.1080/01677063.2017.1338696. [DOI] [PubMed] [Google Scholar]
- 63.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523(7559):231–5 doi 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 64.Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Gothert JR, et al. Endothelial beta-Catenin Signaling Is Required for Maintaining Adult Blood-Brain Barrier Integrity and Central Nervous System Homeostasis. Circulation 2016;133(2):177–86 doi 10.1161/CIRCULATIONAHA.115.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seton-Rogers S Tumour suppressors: Digging deeper into p53’s functions. Nat Rev Cancer 2017;17(12):706–7 doi 10.1038/nrc.2017.108. [DOI] [PubMed] [Google Scholar]
- 66.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 2013;210(10):2057–69 doi 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ham SW, Jeon HY, Jin X, Kim EJ, Kim JK, Shin YJ, et al. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ 2018. doi 10.1038/s41418-018-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, et al. Regulation of PTEN transcription by p53. Mol Cell 2001;8(2):317–25. [DOI] [PubMed] [Google Scholar]
- 69.Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacol Ther 2015;152:54–62 doi 10.1016/j.pharmthera.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 2009;15(19):6002–7 doi 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 71.Kohanbash G, Carrera DA, Shrivastav S, Ahn BJ, Jahan N, Mazor T, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest 2017;127(4):1425–37 doi 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X, Rao A, Sette P, Deibert C, Pomerantz A, Kim WJ, et al. IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro Oncol 2016;18(10):1402–12 doi 10.1093/neuonc/now061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med 2018;24(8):1192–203 doi 10.1038/s41591-018-0095-6. [DOI] [PubMed] [Google Scholar]
- 74.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery 2013;72(6):1031–8; discussion 8–9 doi 10.1227/NEU.0b013e31828cf945. [DOI] [PubMed] [Google Scholar]
- 75.Zhai L, Lauing KL, Chang AL, Dey M, Qian J, Cheng Y, et al. The role of IDO in brain tumor immunotherapy. J Neurooncol 2015;123(3):395–403 doi 10.1007/s11060-014-1687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holtzhausen A, Zhao F, Evans KS, Tsutsui M, Orabona C, Tyler DS, et al. Melanoma-Derived Wnt5a Promotes Local Dendritic-Cell Expression of IDO and Immunotolerance: Opportunities for Pharmacologic Enhancement of Immunotherapy. Cancer Immunol Res 2015;3(9):1082–95 doi 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004;4(10):762–74 doi 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 78.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 2012;18(22):6110–21 doi 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nature Reviews Cancer 2016;16:447 doi 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 80.Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial Stimulation of Glioblastoma Invasion Involves Epidermal Growth Factor Receptor (EGFR) and Colony Stimulating Factor 1 Receptor (CSF-1R) Signaling. Molecular Medicine 2012;18(1):519–27 doi 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. CSF1 receptor signaling is necessary for microglia viability, which unmasks a cell that rapidly repopulates the microglia-depleted adult brain. Neuron 2014;82(2):380–97 doi 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature medicine 2013;19(10):1264–72 doi 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014;344(6190):1396–401 doi 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Furnari FB, Cloughesy TF, Cavenee WK, Mischel PS. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat Rev Cancer 2015;15(5):302–10 doi 10.1038/nrc3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nature reviews Neurology 2015;11(9):504–14 doi 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-Oncology 2015;17(8):1064–75 doi 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garber ST, Hashimoto Y, Weathers S-P, Xiu J, Gatalica Z, Verhaak RGW, et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro-Oncology 2016;18(10):1357–66 doi 10.1093/neuonc/now132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahmoudi M, Farokhzad OC. Cancer immunotherapy: Wound-bound checkpoint blockade. Nature Biomedical Engineering 2017;1:0031 doi 10.1038/s41551-017-0031. [DOI] [Google Scholar]
- 89.Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 2017;8(53):91779–94 doi 10.18632/oncotarget.21586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Velicu S, Han Y, Ulasov I, Brown IE, El Andaloussi A, Gajewski TF, et al. Cross-priming of T cells to intracranial tumor antigens elicits an immune response that fails in the effector phase but can be augmented with local immunotherapy. J Neuroimmunol 2006;174(1–2):74–81 doi 10.1016/j.jneuroim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 91.Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med 2013;210(13):2803–11 doi 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.King GD, Muhammad AK, Larocque D, Kelson KR, Xiong W, Liu C, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther 2011;19(10):1793–801 doi 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sasaki K, Zhu X, Vasquez C, Nishimura F, Dusak JE, Huang J, et al. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res 2007;67(13):6451–8 doi 10.1158/0008-5472.CAN-06-3280. [DOI] [PubMed] [Google Scholar]
- 94.Samaha H, Pignata A, Fousek K, Ren J, Lam FW, Stossi F, et al. A homing system targets therapeutic T cells to brain cancer. Nature 2018;561(7723):331–7 doi 10.1038/s41586-018-0499-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]