Abstract
Peptide hormones and neurotransmitters involved in reproduction and growth have been studied extensively in certain gastropod molluscs, such as Lymnaea stagnalis and Aplysia californica. The present study employs antisera that have been used to study peptidergic neurons in those species to probe the central nervous system of another gastropod, Biomphalaria alexandrina, an intermediate host of the parasitic trematode that causes schistosomiasis in humans. Whole mount preparations of central ganglia were stained immunohistochemically, and several populations of neurons appeared to be homologous to those forming the neuroendocrine axis that has been previously described in L. stagnalis. These cells include the caudodorsal cells and the light green and canopy cells, which produce hormones that regulate ovulation and growth, respectively. Other populations of cells containing APGWamide, FMRFamide and/or related peptides are consistent with ones that innervate the penis in L. stagnalis and other gastropods. Identification of neurons that might be responsible for the control of reproduction and growth in Biomphalaria provides an important initial step toward the development of novel methods of disease control and pest management directed toward reducing snail populations.
Keywords: neuropeptide, neuroendocrine, reproduction, growth, schistosomiasis
1. INTRODUCTION
Schistosomiasis is a major public health concern in parts of Asia, Africa, and South America. The transmission of this parasitic disease has been recorded in 78 countries and recently the World Health Organization (WHO) estimated that about 206 million people required preventive chemotherapy, although less than 50% of this number was reported to have been treated (WHO, 2011). The different trematode species that are responsible for the forms of the disease that infect humans all require an obligatory larval period within a gastropod host (Fenwick et al., 2006). Snails of the genus Biomphalaria serve as the intermediate hosts in many ecosystems. In addition to this significant role in infectious disease transmission, Biomphalaria species have also been proposed as model organisms for the study of human reproductive disorders (Kaur et al., 2016) and potential risks of water pollution (Habib et al., 2016). Together, these studies have created considerable interest in Biomphalaria within the biomedical community.
In contrast to other gastropod species, such Lymnaea stagnalis and Aplysia californica, which have been studied for decades as model organisms in neuroscience (Kandel, 1979; Benjamin et al., 1985; Scheller and Schaefer, 1985; Chase, 2002), much about the nervous system of Biomphalaria species remains unknown. Fortunately, it is expected that the sequencing of the entire genome of Biomphalaria glabrata (Adema et al., 2017) and the publication of the nervous system transcriptome from Biomphalaria alexandrina (Mansour et al., 2017) should greatly facilitate future work. Furthermore, the nervous systems of B. alexandrina and of B. glabrata have been described using immunohistochemical techniques to localize aminergic transmitters that may be involved in reproduction, growth and host-parasite interactions (Delgado et al., 2012; Omran, 2012; Vallejo et al., 2014; Habib et al., 2015). These studies showed that the organization of the central ganglia and even the locations and transmitter contents of many populations of neurons were similar to those in other gastropod species, especially L. stagnalis. These findings set a foundation for the use of antibodies raised against peptidergic neurons involved in the reproduction and growth of L. stagnalis and A. californica to further characterize the nervous system of Biomphalaria.
In the present study, we have investigated the nervous system of B. alexandrina, which has a long history as the vector for transmission of the trematode Schistosoma mansoni in Egypt (Barakat, 2013; Haggag et al., 2017). Specifically, in the current study, we use antibodies raised against α-caudodorsal cell peptide (αCDCP) from L. stagnalis and egg-laying hormone (ELH) from A. californica to localize cells that might secrete hormones involved in the control of ovulation (Stuart and Strumwasser, 1980; Van Minnen et al., 1988). Antibodies against molluscan insulin-like peptides (MIPs) from the light green and canopy cells of L. stagnalis were used to identify cells that might control growth (Smit et al., 1988). These antisera have previously been used to identify potential neurosecretory cells in a variety of other gastropods (Van Minnen and Schallig, 1990; Griffond et al., 1992; Croll et al., 1993; Ram et al., 1998), including B. glabrata (Van Minnen et al., 1992). Antibodies against the neuropeptide APGWamide have also been used to identify cells which are involved in male copulation in other hermaphroditic gastropods (Smit et al., 1992; Chase and Li, 1993; Fan et al., 1997; De Lange et al., 1998; De Lange and Van Minnen, 1998; Koene et al. 1999) Finally, antibodies against FMRFamide, previously shown to stain other populations of neurons innervating the penis of L. stagnalis (De Lange et al., 1998), were also used. In addition to the studies on various gastropods listed above, these different antibodies have also been used to tentatively localize peptides in both bivalve (Croll et al., 1993; Croll et al., 1995; Smith et al., 1997) and cephalopod (Henry and Zatylny, 2002; Di Cristo et al., 2005) molluscs. For a review of the functions and localizations of the peptides across the molluscs, see also Di Cosmo and Polese (2013). For a review of how some of these neuropeptides might control the detection of olfactory cues involved in the reproduction of cephalopods, see Polese et al. (2015).
The positions and morphologies of the various immunoreactive neurons found in the present study suggest homology with neurons controlling reproduction and growth in L. stagnalis and therefore also suggest that the neurons described here might have similar functions in Biomphalaria. A more extensive understanding of the nervous system of Biomphalaria will form a solid base for investigations of host-parasite interactions, reproductive behaviour and the development of methods for snail population control with the aim of improving public health where schistosomiasis is prevalent.
2. MATERIALS AND METHODS
2.1. Animals
Individuals (12-15 mm in shell diameter) were taken from a breeding laboratory colony of B. alexandrina. These snails were all descendants of specimens that were collected from freshwater habitats in the Giza governorate, Egypt (population described in Vallejo et al., 2014). Upon arrival in Halifax, snails were fed romaine lettuce ad libitum and were afforded unrestricted breeding opportunities in large glass aquaria. The colony was maintained on a 14:10 light:dark schedule and housed at ambient room temperature in the laboratory.
2.2. Tissue preparation
Snails were dissected following anesthetization in 50 mmol L−1 MgCl2 for 30 minutes (Ross and Ross, 2009). The shell was crushed by applying light pressure to its lateral surface and removed in pieces. Animals were then pinned to a Sylgard silicone elastomer base (Dow Corning, Midland, MI USA) in a Petri dish filled with the MgCl2 solution (Fig. 1A). The central ganglia were then removed and transferred to a smaller Petri dish, also containing Sylgard and normal snail saline (composition in mmol L−1 is: NaCl 51.3, KCl 1.7, MgCl2 1.5, CaCl2 4.1, NaHCO3 1.8, pH 7.8). The pedal commissure was cut to allow the cerebral ganglia to lay flat for viewing in wholemount (Fig. 1B). The cerebral ganglia were the exclusive focus of the present study as they contain the major neuroendocrine centres that are responsible for reproduction and growth in L. stagnalis (Geraerts, 1976; Smit et al., 1988; Croll and Van Minnen, 1992; Smit et al., 1992). The ganglia were then pinned to the base in the dish and connective tissue was removed by gently scraping their surfaces with the tip of a hypodermic needle. The saline was removed and replaced with 4% paraformaldehyde in phosphate buffered saline (PBS: 100 mM Na2HPO4; 147 mM NaCl; pH 7.4). Following 8-12 hours of fixation, the tissue was rinsed with PBS and incubated a further 8-12 hours in blocking solution (PBS containing: 1% bovine serum albumin, 1% normal goat serum, 1% Triton X-100 (Sigma-Aldrich, Mississauga, ON, CAN), and 1% dimethyl sulfoxide (Fisher Scientific, Ottawa, ON, CAN).
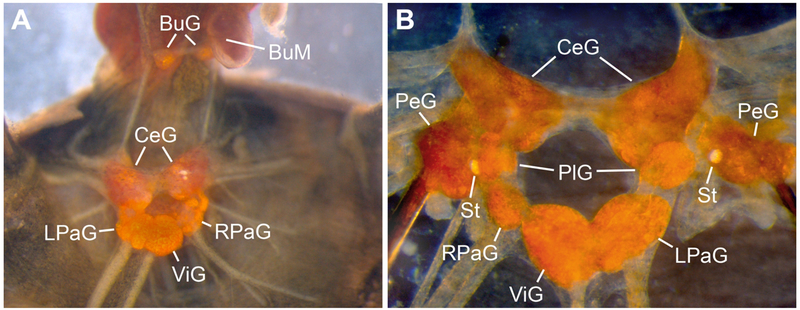
Figure 1.
The central nervous system (CNS) of Biomphalaria alexandrina in various stages of dissection. A) Dorsal perspective of a semi-isolated CNS following removal of the esophagus. The pedal ganglia (PeG) are obscured by the overlying cerebral ganglia (CeG). B) Ventral perspective of an isolated CNS with the pedal commissure cut and pedal ganglia pinned laterally (BuG = buccal ganglia; BuM = buccal mass; LPaG = left parietal ganglion; PlG = pleural ganglia; RPaG = right parietal ganglion; St = statocyst; ViG = visceral ganglion).
2.3. Antibodies and immunohistochemical procedures
Tissues were next incubated in primary antibody solutions for one week at 4 °C. Antibodies raised in rabbits against ELH were obtained from J. Blankenship (University of Texas), while rabbit antibodies against αCDCP, APGWamide and MIP were all obtained from J. van Minnen (Vrije Universiteit of Amsterdam). References describing the antigens used for raising each antibody are provided in Table 1. Finally, antibodies raised in rabbits against FMRFamide were obtained commercially from ImmunoStar (Hudson, WI USA, Cat #20091). The dilutions of each of the primary antibodies are also given in Table 1.
Table 1.
Primary antibodies
| Antibody | Dilution | Reference |
|---|---|---|
| Anti-ELH | 1:300 | (Ram et al., 1998) |
| Anti-αCDCP | 1:250 | (Van Minnen et al., 1992) |
| Anti-APGWamide | 1:200 | (Croll and Van Minnen, 1992) |
| Anti-MIP | 1:200 | (Van Minnen and Schallig, 1990) |
| Anti-FMRFamide | 1:200 | (Lehman and Price, 1987; Voronezhskaya and Elekes, 2003; Kononenko and Zhukov, 2005) |
Following primary incubation, the ganglia were washed four times over 8-12 hours in fresh blocking solution. Tissue was then incubated for 5 days at 4 °C in darkness in either goat-antirabbit antibodies conjugated to AlexaFluor488 (Molecular Probes, Eugene, OR, USA; Cat # A11008) or goat-anti-rabbit conjugated to Cy3 (Jackson ImmunoResearch, West Grove, PA, USA; Cat #111-165-003), both diluted 1:200 in blocking solution. The ganglia were then washed for 6-8 hours in blocking solution before being transferred to glycerol mounting medium (25% 0.05M Tris buffer in 75% glycerol with the addition of 2% n-propyl-gallate to minimize photobleaching).
2.4. Imaging
All cerebral ganglia were mounted on glass slides for initial imaging with a Leica DM 4000B fluorescent microscope. Images were obtained using a Leica D500 model digital camera and imported into Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA). These images were used to provide much of the information on cell locations, numbers and sizes reported here. Further imaging of selected preparations was performed using a Zeiss LSM 510 (Carl Zeiss Inc, Germany) confocal microscope to obtain optical sections in 0.5–1.5 μm intervals. These sections were arranged as maximum intensity projections and the images were used to map cells and produce representative images using ImageJ software (Schindelin et al., 2012; Rueden et al., 2017), ZEN 2009 software (Carl Zeiss Inc, Germany) and Adobe Photoshop. Cell measurements were done using ImageJ. Schematic diagrams were created using Adobe Illustrator (Adobe Creative Suite 7).
2.5. Controls
Tests for the specificity of each of the primary antibodies in gastropods are described in references enumerated in Table 1. The specificity of the secondary antibodies was tested by omitting the primary antibody incubations from the procedures. No significant fluorescence was observed in any control preparations.
3. RESULTS
All results reported here are based on observations of 5-10 specimens tested for each antibody.
3.1. MIP
Cells exhibiting molluscan insulin-related peptide (MIP) like-immunoreactivity (LIR) were located almost exclusively in bilateral clusters lying in medial portions of the cerebral ganglia, immediately adjacent to the cerebral commissure (Fig. 2A). These cells numbered up to about 35 per hemiganglion and ranged widely in size from less than 15 μm up to about 50 μm in diameter with a median diameter of approximately 26 μm. A prominent tract of axons projected from each cluster to the ipsilateral medial lip nerve. Upon entering the nerve, the tight fascicle of axons spread out along the outer surface of the nerve and appeared to form numerous varicose terminals (Fig. 2B), characteristic of neurohaemal release sites previously described in other pulmonate gastropods (Van Heumen and Roubos, 1991; Sonetti et al., 1992; Khan et al., 1999). In addition to the large, medial clusters of MIP-LIR neurons, each cerebral ganglion also possessed a single immunoreactive cell (ranging 35-44 μm in diameter) generally positioned midway along its lateral margin (Fig. 2A). A prominent axon could be seen projecting from each of these lateral cells toward the medial cluster. Similar neurons, which are referred to as canopy cells, have been describe in other pond snails (Van Minnen et al., 1979; Sonetti et al., 1992). While these neurons were laterally positioned in almost all preparations, Fig. 2A shows what appears to be an ectopic canopy cell, with its prominent medial axon, located more centrally in the right cerebral ganglion. In addition, a few, fainter and smaller immunoreactive neurons were scattered in other regions of the cerebral ganglia.
Figure 2.
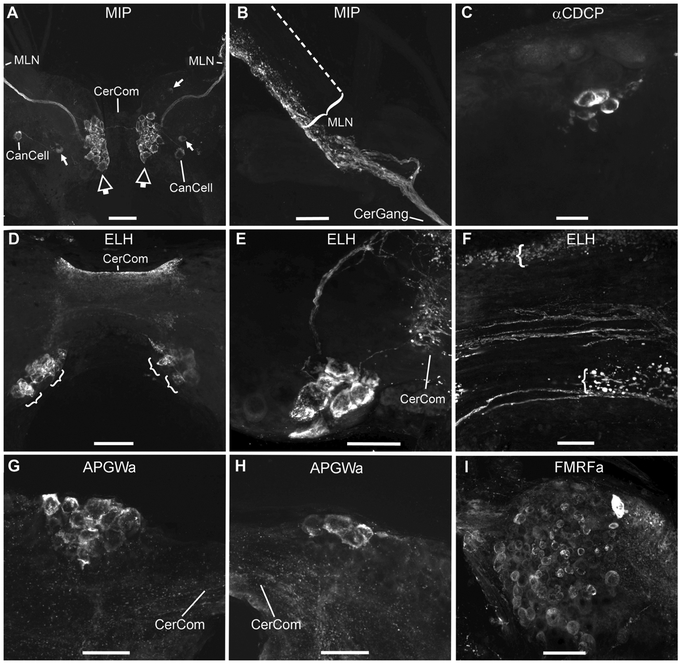
Neuropeptides in the cerebral ganglia of Biomphalaria alexandrina. A: Molluscan insulin-related peptide (MIP)-like immunoreactivity is located in symmetric clusters of medial neurons (larger, hollow arrows), adjacent to cerebral commissure (CerCom). A bundle of axons projects from each cluster to the median lip nerve (MLN). A single canopy cell (CanCell) was also labelled in each ganglion. On the left, the cell assumed its characteristic lateral position whereas on the right, the cell was located more centrally. A few additional, immunoreactive cells (smaller arrows) are scattered in other regions of the ganglia. B: After the tight bundle of MIP-LIR axons exits the cerebral ganglion (CerGang), it projects to the lateral surface of the MLN where it appears to form a neurohaemal region along its length. The dotted line indicates the location of the faint medial edge of the MLN. C: Antibodies against the α-caudodorsal cell peptide (αCDCP) of Lymnaea label a cluster of small cells near the center of the cerebral ganglion. D: Antibodies against the egg laying hormone (ELH) of Aplysia, reveal two bilateral clusters of large neurons (indicated by brackets) posterior to the cerebral commissure (CerCom). The CerCom itself was also intensely fluorescent. E: A higher magnification image of ELH-positive cells projecting into the adjacent to the CerCom which also contains numerous immunoreactive varicosities. F: Another high magnification image of both intact, ELH-positive axons traversing the CerCom and also numerous varicosities on the anterior and posterior margins of the commissure (indicated by brackets), which appear to constitute another neurohaemal region for peptide release. G: A cluster of large APGWamide-like-immunoreactive cells in the left anterior lobe. H: APGWamide-like-immunoreactive neurons in the right anterior lobe of the same snail as shown in G. I: FMRFamide-like-immunoreactivity in numerous small neurons in the left ventral lobe. All images are oriented to present anterior toward the top of the plate and all images, except for I, show dorsal views. Scale bars equal: 100 μm in A and D; 50 μm in B, E, G and H; 25 μm in C and F; and 20 μm in I.
3.2. αCDCP
Clusters of α-caudodorsal cell peptide (αCDCP)-LIR neurons were also reliably located in the cerebral ganglia (Fig. 2C). These cells were relatively few in number (1-14 cells per hemiganglion) and their locations appeared to be somewhat inconsistent, although they were most often positioned near the medial edge of the ganglia and in regions caudal to the cerebral commissure. In a few specimens, cells were located in only the left hemiganglion. In other specimens, cells were located bilaterally with a median count of 5.5 cells in the left hemiganglion and 5 cells in the right. Cell diameters varied considerably, ranging from approximately 15-55 μm with a median diameter of approximately 32 μm. Axons from these cells were generally not well labelled and did not form prominent tracts or neurohaemal release sites.
3.3. ELH
Antibodies raised against Aplysia egg laying hormone (ELH) reliably labelled two closely positioned clusters of medial neurons posterior to the cerebral commissure (Fig. 2D). Together the neurons in these clusters generally totaled 15-20 cells per hemiganglion and ranged widely in size from approximately 10-40 μm with a median diameter of approximately 19 μm. Axons from these cells projected into and appeared to cross the cerebral commissure (Fig. 2E, F). Many of the axons also appeared to terminate in varicosities that were particularly prominent along the anterior and posterior surfaces of the commissure, suggesting that these were sites for neurohaemal release.
3.4. APGWamide
APGWamide-LIR cells were generally located along the anterior margin of each cerebral ganglion, near the cerebral commissure. The numbers of these cells varied greatly from just a couple of cells up to a total of 19 neurons per hemiganglion. The cells were also variably sized between approximately 6-43 μm with a median diameter of about 20 μm. These clusters were either bilaterally symmetric or contained slightly more cells on the left (Fig. 2G) than on the right (Fig. 2H). The cluster size contained a median of 6 cells in the left ganglion and 5.5 in the right ganglion. In some samples, a small population of 3-9 APGWamide-LIR cells were seen on the ventral side of the left ganglion, near the base of the penial nerve (data not shown). These cells were smaller than the other cluster with a median diameter of 8 μm.
3.5. FMRFamide
FMRFamide-LIR cells were abundant throughout the central nervous system and particularly in the cerebral ganglion (not shown). These cells generally appeared to be bilaterally symmetric on the dorsal surface of the cerebral ganglion, but a prominent asymmetry was noted on the ventral surface, with only the left cerebral ganglion possessing a small lobe near the base of the cerebral-pedal connective. This ventral lobe contained approximately 185-225 FMRFamide-LIR cells which were densely packed and relatively small, ranging 4-18 μm in size with a median diameter of about 10 μm (Fig. 2I). Apart from the ventral lobe, the left and right cerebral ganglia appeared to contain bilaterally symmetric populations of approximately 140–236 FMRFamide-LIR cells ranging from 3–45 μm located near or anterior to the cerebral-pedal connective.
4. DISCUSSION
The results described in this report and summarized in Figure 3 suggest that the neurons controlling reproduction and growth in Biomphalaria alexandrina share numerous characteristics with those that have already been studied extensively in Lymnaea (for reviews see Joose, 1988; Smith and Croll, 1998; Koene, 2010; Mukai and Morishita, 2017). The cells contain similar neuropeptides as demonstrated by cross reactivity to directed antibodies, but they also share strikingly similar locations and axonal projections, thus supporting their probable homology (Croll, 1987). In fact, our findings are consistent with a body of previous evidence also suggesting evolutionary conservation of peptidergic neurons involved in reproduction and growth in other gastropods, as described below, and with recent findings of homologous individual cells and cell population containing classical transmitters such as serotonin (Delgado et al., 2012), dopamine (Vallejo et al., 2014), histamine (Habib et al., 2015) and GABA (Vaasjo et al., 2018) in Biomphalaria, Lymnaea and other heterobranch gastropods.
Figure 3.
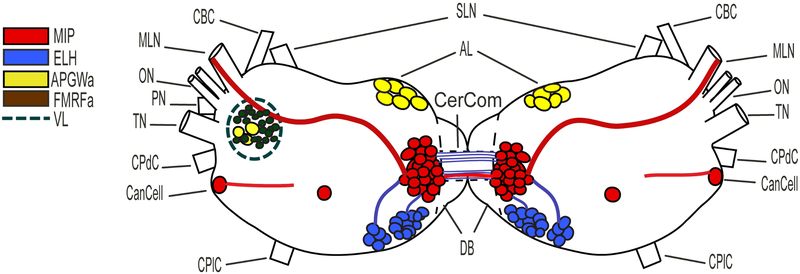
Schematic representation of the cerebral ganglia of B. alexandrina showing the location of the peptides under investigation (each peptide is assigned a unique color, see the key on the left side). MIP and ELH axon terminals are abundant in the median lip nerve and cerebral commissures. Abbreviations: AL: anterior lobe, CanCell: canopy cell, CBC: cerebral-buccal connective, CerCom: cerebral commissure, CPdC: cerebral-pedal connective, CPlC: cerebral-pleural connective, DB: dorsal bodies, MLN: medial lip nerve, On: optic nerve, PN: penial nerve, SLN: superior lip nerve, Tn: tentacular nerve, VL: ventral lobe.
One notable difference, however, between Biomphalaria and Lymnaea is that the nervous system of the former seems to be considerably smaller than that reported for Lymnaea. Previous work has reported peptidergic cell measurements in Lymnaea that were substantially higher than the values reported here (Moed et al., 1989; Geraerts, 1992; De Lange et al., 1997). In the present study, however, we relied exclusively on wholemount immunohistochemistry to label the different cell populations and therefore used only smaller animals, which present less difficulties with penetration and visualization (Croll and Chiasson, 1989). Although we sampled individuals that were well within the body range considered “adult” by others (e.g. Rizk et al., 2012) and which reproduced in our breeding tanks, further maturation may have yielded larger cell sizes and numbers. In fact, specimens of B. alexandrina can grow to 20 mm or more in laboratory colonies (Lotfy et al., 2005). It is therefore likely that snails used in this study were still in the process of maturation and that we would have seen more plentiful and larger cells if the snails had been older, as occurs with other cell populations in gastropods (Croll and Chiasson, 1989; Cash and Carew, 1989).
4.1. Molluscan insulin-like peptides (MIPs)
The first MIP gene was sequenced by Smit et al. (1988) with subsequent work demonstrating a total of five MIPs expressed in the light green cells (LGCs) and canopy cell of Lymnaea (Smit et al., 1988; Meester et al., 1992). Evidence suggests that the 75-100 LGCs per cerebral hemiganglion in that species (Van Minnen and Schallig, 1990; Geraerts et al., 1992) control glycogen storage and shell growth (Geraerts, 1992), probably through the actions of the MIPs (Smit et al., 1988).
The genes for insulins in vertebrates share similar overall organizations with those for MIPs in Lymnaea, with each producing A and B peptide subunits that form the completed proteins, and linking C peptides used for their folding. While the various MIPs in Lymnaea differ with regard to the amino acid sequences of the A and B proteins, the C protein is more highly conserved and an antibody against this sequence appears to label cells expressing all of the MIPs in Lymnaea and other gastropods (Van Minnen and Schallig, 1990). It is this antibody that we used here.
The large clusters of cells that are reactive to this MIP antibody in B. alexandrina appear to be homologous to the LGCs in Lymnaea and the single, lateral MIP-LIR cells observed in the present study are likely homologous to the canopy cells. We observed here the same locations of large MIP-LIR cells lateral to the cerebral commissure, canopy cells along the lateral edges of the ganglia and axons crossing the commissure (although we cannot confirm that these commissural axons project from the canopy cells). We also observed the same tight bundles of axons projecting to the medial lip nerves, which appears to contain the neurohaemal organ used for releasing the MIPs into the haemolymph (Van Heumen and Roubos, 1990). However, we observed only a single large cluster of MIP-LIR cells in each cerebral hemiganglion rather two closely positioned populations of medial and lateral LGCs described in Lymnaea and other basommatophoran snails (Van Minnen and Schallig, 1990).
While this is the first demonstration of LGC homologues in B. alexandrina, they have previously been demonstrated in B. glabrata using the same antibody employed here (Van Minnen and Schallig, 1990). In addition, Khan et al. (1992) demonstrated similar cells in another planorbid snail, Helisoma, using antibodies raised against porcine insulin and subsequent work indicated roles in shell growth and regeneration in that species (Saleuddin et al., 1992). Similarly, Gomot et al. (1992) demonstrated homologous cells in Helix aspersa using antibodies raised against bovine insulin, and showed that they were essential for growth and glucose metabolism in that species as well.
4.2. Ovulation hormones
The family of egg-laying hormone (ELH) genes in Aplysia were first sequenced by Scheller et al. (1983) with later work by Vreugdenhil et al. (1988) sequencing the caudodorsal cell hormone (CDCH) gene of Lymnaea. The ovulation hormones encoded by these genes were found to be 36 amino acids long in both species and share about 50% sequence identity. Ram et al. (1998) subsequently used antibodies raised against an N-terminus sequence of Aplysia ELH to stain the caudodorsal cells (CDCs) and cerebral commissure of Lymnaea. The N-terminus region of Aplysia ELH also shares approximately 50% homology with the putative ovulation hormone from Biomphalaria (Supplemental Table 1), and we therefore used this same antibody here.
The cells that react to antibodies raised against ELH in Biomphalaria are strikingly similar to the caudodorsal cells which synthesize and secrete CDCH in Lymnaea (Geraerts et al., 1983). The cells in both species are positioned caudal to the cerebral commissure in roughly symmetric distributions. In both species, the cells can also be seen projecting into and across the cerebral commissure with its associated neurohaemal area. The two closely located clusters of neurons observed here in Biomphalaria may correspond to the dorsal and ventral CDCs described in Lymnaea (Van Minnen et al., 1988). Similar cells have been described in Helisoma, where they are also implicated in the control of egg laying (Mukai and Saleuddin, 1989; Khan et al., 1990).
In addition to containing the ovulation hormones themselves, their preprohormones from both Lymnaea and Aplysia also produce several smaller peptides that have a variety of functions, such as activating accessory sexual organs or modifying the activities of other central neurons producing the various aspects of the complete egg-laying behaviours (Rothman et al., 1983; Scheller et al., 1983). Van Minnen et al. (1992) previously reported reactivity to an antibody raised against one caudodorsal peptide (αCDCP) in other pulmonates including Helix, Limax and B. glabrata, but provided few details in the latter species. We also saw αCDCP-LIR cells in B. alexandrina, but they were noticeably smaller and less numerous than the ELH-LIR cells. We also observed no sign of a neurohaemal region in the cerebral commissure of B. alexandrina using anti-αCDCP. Our results thus suggest that anti-αCDCP may only react with a small subpopulation of neurons expressing the ovulation hormone in Biomphalaria. We cannot, however, discount the possibility that αCDCP-LIR might represent cross-reactivity with an unrelated peptide expressed in nearby cells.
4.3. APGWamide
Just as staining with antibodies against MIP and ELH revealed neurosecretory cells controlling growth and ovulation in Biomphalaria, our work also demonstrates neurons that likely control aspects of male reproduction. The neuropeptides APGWamide and FMRFamide are both involved with penis control in other gastropods and genes encoding these peptides have been identified in Biomphalaria (Supplemental Table 1). Antibodies raised against each of the tetrapeptides appeared to label homologous neurons in this study. .
In Lymnaea, APGWamide is found predominantly in the asymmetric anterior lobes of the cerebral ganglia and the cells of the larger right lobe have been shown to project to the penis (Croll and Van Minnen, 1992; Smit et al., 1992). Fine wire electrical recordings of these cells in freely behaving snails demonstrate that they are active during copulation (De Boer et al., 1997), while other studies have demonstrated that APGWamide inhibits contractions of the penis retractor muscle of Lymnaea (Croll et al., 1991). A large cluster of APGWamide-LIR neurons in the right cerebral ganglion also innervates the penial complex of Helix (Li and Chase, 1995), and again, a variety of techniques have been used to implicate APGWamide in the control of penial movements, specifically eversion of the genitalia in preparation for copulation (Koene et al., 2000). Other studies also indicate roles for asymmetric anteromedial clusters of APGWamide-LIR neurons in penial control in the pulmonate slugs Arion and Limax and the marine opisthobranch Aplysia (Fan et al., 1997; De Lange and Van Minnen, 1998). In all these dextral species, the larger right cluster of cerebral neurons was located ipsilateral to the penis (Baker, 1905).
The majority of APGWamide-LIR cells in the cerebral ganglia of Biomphalaria lay in regions corresponding to the anterior lobes of Lymnaea and other gastropods (De Lange and Van Minnen, 1998). Our findings may also suggest a possible asymmetry with slightly more cells on the left side, consistent with the fact that the penis of the sinistral snail Biomphalaria is located on the left side (Mandahl-Barth, 1957). However, our evidence for an asymmetric distribution in Biomphalaria is weak. As discussed above, the specimens examined in the present study represented young adults, and it is possible that a greater asymmetry may develop with further maturation. Nevertheless, previous research has shown that the APGWamide-LIR cells were also symmetrically distributed in the cerebral ganglia in certain other gastropods, like the caenogastropod Littorina and the freshwater pulmonate Bulinus, although in the latter species APGWamidergic fibres were nonetheless still observed to innervate the penial complex (De Lange and Van Minnen, 1998). Thus, the APGWamide-LIR cells described here may be involved in penis control, regardless of the symmetry of their distribution within the cerebral ganglia.
In addition to the large APGWamide-LIR cells in the anteromedial regions of the cerebral ganglia, further immunoreactivity was detected in a small cluster of cells near the lateral edge of the left cerebral ganglion, in a region corresponding to the ventral lobe (see below). Croll and Van Minnen (1992) similarly reported a small number of APGWamide-LIR cells in the ventral lobe of Lymnaea ipsilateral to the penis.
4.4. FMRFamide
FMRFamide and related peptides (many of which react to the antibodies used here) have been detected in numerous gastropod genera and are widely distributed within the body (reviewed by Greenberg and Price, 1989; Lopez-Vera et al., 2008). In several species, FMRFamide and related peptides have been found in high concentrations in both the brain and reproductive tract (e.g., H. aspersa [Lehman and Price, 1987]; Melampus bidentatus [Khan et al., 1999]). Studies of B. glabrata and Lymnaea have demonstrated that FMRFamide activates muscles which control movement of the preputium and penis (Van Golen et al., 1995; Fong et al. 2005) and in H. aspersa, FMRFamide has been implicated in the control of male courtship behaviour (Li and Chase, 1995).
In the present study we found that FMRFamide-LIR was widespread in the cerebral ganglia and likely plays several roles in this species. Of particular note, however, was a concentrated population of cerebral neurons that likely innervates the penial complex. In Lymnaea, a population of numerous, small, FMRFamide-LIR neurons is located in the ventral lobe of the right cerebral ganglion (Schot and Boer, 1982; Bright et al., 1993), ipsilateral to the penis, which they innervate (Smit et al., 1992; De Boer et al., 1996). In Biomphalaria, we likewise observed a ventral lobe containing numerous FMRFamide-LIR cells ipsilateral to the penis, which is on the left side in this species as discussed above. No discernible ventral lobe was detected on the side contralateral to the penis in either species. Also, similar to Lymnaea, the cells of the ventral lobe of Biomphalaria generally were uniformly small and appeared to contain a subpopulation of APGWamide-LIR cells (see above). Although axonal tracing studies have yet to be conducted on B. alexandrina, cells of the left ventral lobe of B. glabrata have been confirmed to innervate the penial complex (Delgado et al., 2012).
4.5. Conclusions
Decades of research using Aplysia and Lymnaea as model organisms have informed our understanding of the neural substrates underlying growth and reproduction in gastropods. Comparisons between these species have indicated a high degree of conservation of genes, peptides and behaviours and have suggested generalizations applicable to a wide range of other gastropods, including Biomphalaria. To date, B. glabrata has been the focus of several studies regarding both classical neurotransmitters and peptides (Roubos and Van de Ven, 1987; Van Minnen and Schallig, 1990; Van Minnen et al., 1992; Fong et al., 2005; Delgado et al., 2012). In fact, homologues to genes encoding each of the peptides examined here have now been reported in B. glabrata (Wang et al., 2017; see also Supplementary Table 1). Recently, however, there has been an interest in building on this work toward a broader understanding of Biomphalaria as a genus, given its importance to global public health. To this end, several studies of classical neurotransmitters have also included B. alexandrina (Vallejo et al. 2014; Habib et al., 2015; Vaasjo et al., 2018). Generally, distribution patterns are similar, but not identical, in the two species. Here we have described several neurons within the cerebral ganglia of B. alexandrina and conclude, based on their locations, morphologies and immunoreactivity that they are likely homologous to populations previously described in other gastropods, like Lymnaea. The presence of some of these peptides has already been inferred from a recent transcriptome analysis of this species (Mansour et al., 2017; see also Supplementary Table 1). With such striking similarities in the anatomy and biochemistry of these cells, we further hypothesized that the physiology and functions of these cells are also well conserved. We anticipate that this work will form a solid foundation for rapid advances in understanding the mechanisms controlling reproduction and growth in this species and in Biomphalaria as a genus.
Supplementary Material
Acknowledgments
FUNDING
Funding was provided by Discovery Grant #38863 from the Natural Sciences and Engineering Research Council (NSERC) of Canada to RPC, US National Science Foundation HRD-1137725 and OISE 1545803 to MWM, and U.S.-Egypt Science and Technology (S&T) Joint Fund 2000007152; Science and Technology Development Fund (STDF, Egypt): USC17–188 to MWM and MRH.
Footnotes
DECLARATIONS OF INTEREST
None
REFERENCES
- Abdraba AM, Saleuddin ASM 2000. Localization and immunological characterization of insulin-like peptide(s) in the land snail Otala lactea (Mollusca: Pulmonata). Can. J. Zool. 78, 1515–1526. [Google Scholar]
- Adema CM, Hillier LW, Jones CS, Loker ES, Knight M, Minx P, Oliveira G, Raghavan N, Shedlock A, Do Amaral LR, 2017. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Commun. 8, 15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, 1905. Notes on the genitalia of Lymnaea. Am. Nat. 39, 665–679. [Google Scholar]
- Barakat RM, 2013. Epidemiology of schistosomiasis in Egypt: Travel through time. J. Adv. Res. 4, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin PR, Elliott CJ, Ferguson GP, 1985. Neural network analysis in the snail brain In: Selverston A (Ed.), Model Neural Networks and Behavior. Springer, pp. 87–108. [Google Scholar]
- Bright K, Kellett E, Saunders SE, Brierley M, Burke JF, Benjamin PR, 1993. Mutually exclusive expression of alternatively spliced FMRFamide transcripts in identified neuronal systems of the snail Lymnaea. J. Neurosci. 13, 2719–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash D, Carew TJ 1989. A quantitative analysis of the development of the central nervous system in juvenile Aplysia californica. J. Neurobiol, 20: 25–47. [DOI] [PubMed] [Google Scholar]
- Chase R, 2002. Behavior and Its Neural Control in Gastropod Molluscs. Oxford University Press, 336 pp. [Google Scholar]
- Chase R, Li G, 1993. Mesocerebral neurons and their role in the control of mating behaviour. Neth. J. Zool. 44, 212–222. [Google Scholar]
- Croll RP, 1987. Identified neurons and cellular homologies In: Ali MA (Ed.), Nervous Systems in Invertebrates. Springer, pp. 41–59. [Google Scholar]
- Croll RP, Chiasson BJ, 1989. Postembryonic development of serotonin-like immunoreactivity in the central nervous system of the snail, Lymnaea stagnalis. J. Comp. Neurol. 280, 122–142. [DOI] [PubMed] [Google Scholar]
- Croll RP, Nason J, Van Minnen J, 1993. Characterization of central neurons in bivalves using antibodies raised against neuropeptides involved in gastropod egg-laying behavior. Invertebr. Repro. Dev. 24, 161–168. [Google Scholar]
- Croll RP, Too CKL 1995. Detection of FMRFamide-like immunoreactivities in the sea scallop Placopecten magellanicus by immunohistochemistry and Western blot analysis. Cell Tiss. Res. 281, 295–304. [DOI] [PubMed] [Google Scholar]
- Croll RP, Van Minnen J, 1992. Distribution of the peptide Ala-Pro-Gly-Trp-NH2 (APGWamide) in the nervous system and periphery of the snail Lymnaea stagnalis as revealed by immunocytochemistry and in situ hybridization. J. Comp. Neurol. 324, 567–574. [DOI] [PubMed] [Google Scholar]
- Croll RP, Van Minnen J, Kits KS, Smit AB, 1991. APGWamide: Molecular, histological and physiological examination of a novel neuropeptide involved with reproduction in the snail, Lymnaea stagnalis In: Molluscan Neurobiology. North Holland Publishing, pp. 248–254. [Google Scholar]
- De Boer PACM, Ter Maat A, Pieneman AW, Croll RP, Kurokawa M, Jansen RF, 1997. Functional role of peptidergic anterior lobe neurons in male sexual behavior of the snail Lymnaea stagnalis. J. Neurophysiol. 78, 2823–2833. [DOI] [PubMed] [Google Scholar]
- De Boer PACM, Jansen R, Ter Maat A, 1996. Copulation in the hermaphroditic snail Lymnaea stagnalis: A review. Invertebr. Repro. Dev. 30, 167–176. [Google Scholar]
- De Lange RPJ, De Boer PACM, Ter Maat A, Tensen CP, Van Minnen J, 1998. Transmitter identification in neurons involved in male copulation behavior in Lymnaea stagnalis. J. Comp. Neurol. 395, 440–449. [DOI] [PubMed] [Google Scholar]
- De Lange RPJ, Van Golen FA, Van Minnen J, 1997. Diversity in cell specific coexpression of four neuropeptide genes involved in control of male copulation behaviour in Lymnaea stagnalis. Neuroscience 78, 289–299. [DOI] [PubMed] [Google Scholar]
- De Lange RPJ, Van Minnen J, 1998. Localization of the neuropeptide APGWamide in gastropod molluscs by in situ hybridization and immunocytochemistry. Gen. Comp. Endocrinol. 109, 166–174. [DOI] [PubMed] [Google Scholar]
- Delgado N, Vallejo D, Miller MW, 2012. Localization of serotonin in the nervous system of Biomphalaria glabrata, an intermediate host for schistosomiasis. J. Comp. Neurol. 520, 3236–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cosmo A, Polese G, 2013. Molluscan Bioactive Peptides In: Kastin AJ (Ed.), Handbook of Biologically Active Peptides (Second Edition), Academic Press, pp. 276–286. [Google Scholar]
- Di Cristo C, Van Minnen J, Di Cosmo A 2005. The presence of APGWamide in Octopus vulgaris: a possible role in the reproductive behavior. Peptides 26, 53–62. [DOI] [PubMed] [Google Scholar]
- Fan X, Croll RP, Wu B, Fang L, Shen Q, Painter SD, Nagle GT, 1997. Molecular cloning of a cDNA encoding the neuropeptides APGWamide and cerebral peptide 1: Localization of APGWamide-like immunoreactivity in the central nervous system and male reproductive organs of Aplysia. J. Comp. Neurol. 387, 53–62. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Rollinson D, Southgate V, 2006. Implementation of human schistosomiasis control: Challenges and prospects. Adv. Parasitol. 61, 567–622. [DOI] [PubMed] [Google Scholar]
- Fong PP, Olex AL, Farrell JE, Majchrzak RM, Muschamp JW, 2005. Induction of preputium eversion by peptides, serotonin receptor antagonists, and selective serotonin reuptake inhibitors in Biomphalaria glabrata. Invertebr. Biol. 124, 296–302. [Google Scholar]
- Geraerts WPM, 1992. Neurohormonal control of growth and carbohydrate metabolism by the light green cells in Lymnaea stagnalis. Gen. Comp. Endocrinol. 86, 433–444. [DOI] [PubMed] [Google Scholar]
- Geraerts WPM, Cheeseman P, Ebberink RHM, Nuyt K, Hogenes TM, 1983. Partial purification and characterization of the ovulation hormone of the freshwater pulmonate snail Lymnaea stagnalis. Gen. Comp. Endocrinol. 51, 471–476. [DOI] [PubMed] [Google Scholar]
- Geraerts WPM, Smit AB, Li KW, Hordijk PL, 1992. The Light Green Cells of Lymnaea: A neuroendocrine model system for stimulus-induced expression of multiple peptide genes in a single cell type. Experientia 48, 464–473. [DOI] [PubMed] [Google Scholar]
- Geraerts WPM, 1976. The control of ovulation in the hermaphroditic freshwater snail Lymnaea stagnalis by the neurohormone of the caudodorsal cells. Gen. Comp. Endocrinol. 28, 350–357. [DOI] [PubMed] [Google Scholar]
- Gomot A, Gomot L, Marchand C-R, Colard C, Bride J, 1992. Immunocytochemical localization of insulin-related peptide(s) in the central nervous system of the snail Helix aspersa müller: Involvement in growth control. Cell. Mol. Neruobiol. 12, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MJ, Price DA, 1989. Relationships among the FMRFamide-like peptides. Prog. Brain Res. 92, 25–37. [DOI] [PubMed] [Google Scholar]
- Griffond B, Van Minnen J, Colard C, 1992. Distribution of αCDCP-immunoreactive neurons in the central nervous system of the snail Helix aspersa. Reprod. Nutr. Dev. 32, 113–121. [DOI] [PubMed] [Google Scholar]
- Habib MR, Mohamed AH, Osman GY, El-Din ATS, Mossalem HS, Delgado N, Torres G, Rolón-Martínez S, Miller MW, Croll RP, 2015. Histamine immunoreactive elements in the central and peripheral nervous systems of the snail, Biomphalaria spp., intermediate host for Schistosoma mansoni. PloS one 10, e0129800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib MR, Mohamed AH, Osman GY, Mossalem HS, El-Din ATS, Croll RP, 2016. Biomphalaria alexandrina as a bioindicator of metal toxicity. Chemosphere 157, 97–106. [DOI] [PubMed] [Google Scholar]
- Haggag AA, Rabiee A, Elaziz KMA, Gabrielli AF, Hay RA, Ramzy RM, 2017. Mapping of Schistosoma mansoni in the Nile Delta, Egypt: Assessment of the prevalence by the circulating cathodic antigen urine assay. Acta Trop. 167, 9–17. [DOI] [PubMed] [Google Scholar]
- Henry J, Zatylny C 2002. Identification and tissue mapping of APGWamide-related peptides in Sepia officinalis using LC-ESI-MS/MS. Peptides 23, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Joosse J, 1988. The hormones of molluscs In: Laufer H, Downer RGH (Eds.), Endocrinology of Selected Invertebrate Types. Liss, pp. 89–140. [Google Scholar]
- Kandel ER, 1979. Behavioral Biology of Aplysia: A Contribution to the Comparative Study of Opisthobranch Molluscs. W. H. Freeman, 463 pp. [Google Scholar]
- Kaur S, Baynes A, Lockyer AE, Routledge EJ, Jones CS, Noble LR, Jobling S, 2016. Steroid androgen exposure during development has no effect on reproductive physiology of Biomphalaria glabrata. PloS One 11, e0159852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HR, Ashton ML, Mukai ST, Saleuddin A, 1990. The effects of mating on the fine structure of neurosecretory caudodorsal cells in Helisoma duryi (Mollusca). Can. J Zool. 68, 1233–1240. [Google Scholar]
- Khan HR, Price DA, Doble KE, Greenberg MJ, Saleuddin ASM, 1999. Osmoregulation and FMRFamide-related peptides in the salt marsh snail Melampus bidentatus (Say) (Mollusca: Pulmonata). Biol. Bull. 196, 153–162. [DOI] [PubMed] [Google Scholar]
- Khan HR, Griffond B, Saleuddin A, 1992. Insulin-like peptide(s) in the central nervous system of the snail Helisoma duryi. Brain Res. 580, 111–114. [DOI] [PubMed] [Google Scholar]
- Koene JM, 2010. Neuro-endocrine control of reproduction in hermaphroditic freshwater snails: Mechanisms and evolution. Front. Behav. Neurosci. 4, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene JM, Jansen RF, Ter Maat A, Chase R, 1999. An in vivo electrophysiological study of mating behaviour in the snail Helix aspersa. Invertebr. Repro. Dev. 36, 123–127. [Google Scholar]
- Koene JM, Jansen RF, Ter Maat A, Chase R, 2000. A conserved location for the central nervous system control of mating behaviour in gastropod molluscs: Evidence from a terrestrial snail. Journal of Exp. Biol. 203, 1071–1080. [DOI] [PubMed] [Google Scholar]
- Kononenko NL, Zhukov VV, 2005. Neuroanatomical and immunocytochemical studies of the head retractor muscle innervation in the pond snail, Lymnaea stagnalis L. Zoology 108, 217–237. [DOI] [PubMed] [Google Scholar]
- Lehman HK, Price DA, 1987. Localization of FMRFamide-like peptides in the snail Helix aspersa. J. Exp. Biol. 131, 37–53. [DOI] [PubMed] [Google Scholar]
- Li G, Chase R, 1995. Correlation of axon projections and peptide immunoreactivity in mesocerebral neurons of the snail Helix aspersa. J. Comp. Neurol. 353, 9–17. [DOI] [PubMed] [Google Scholar]
- Lopez-Vera E, Aguilar MB, Heimer de la Cotera EP, 2008. FMRFamide and related peptides in the phylum mollusca. Peptides 29, 310–317. [DOI] [PubMed] [Google Scholar]
- Lotfy WM, DeJong RJ, Black BS, Loker ES, 2005. Specific identification of Egyptian Biomphalaria species using a polymerase chain reaction based on nuclear and mitochondrial loci. Mol. Cell. Probes 19, 21–25. [DOI] [PubMed] [Google Scholar]
- Mandahl-Barth G, 1957. Intermediate hosts of Schistosoma: African Biomphalaria and Bulinus: 1. Bull. World Health Organ. 16, 1103–1163. [PMC free article] [PubMed] [Google Scholar]
- Mansour TA, Habib MR, Rodríguez LCV, Vázquez AH, Alers JM, Ghezzi A, Croll RP, Brown CT, Miller MW, 2017. Central nervous system transcriptome of Biomphalaria alexandrina, an intermediate host for schistosomiasis. BMC Res. Notes 10, 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meester I, Ramkema MD, Van Minnen J, Boer HH, 1992. Differential expression of four genes encoding molluscan insulin-related peptides in the central nervous system of the pond snail Lymnaea stagnalis. Cell Tissue Res. 269, 183–188. [DOI] [PubMed] [Google Scholar]
- Moed PJ, Pieneman AW, Bos NPA, Ter Maat A, 1989. The role of cAMP in regulation of electrical activity of the neuroendocrine caudodorsal cells of Lymnaea stagnalis. Brain Res. 476, 298–306. [DOI] [PubMed] [Google Scholar]
- Mukai ST, Morishita F, 2017. Physiological functions of gastropod peptides and neurotransmitters In: Saleuddin S, Mukai S (Eds.), Physiology of Molluscs. Apple Academic Press, pp. [Google Scholar]
- Mukai ST, Saleuddin ASM, 1989. Mating increases the synthetic activity of the neurosecretory caudodorsal cells of Helisoma duryi (Mollusca: Pulmonata). Can. J. Zool. 67, 2363–2367. [Google Scholar]
- Omran NESES, 2012. Testosterone, gonadotropins and androgen receptor during spermatogenesis of Biomphalaria alexandrina snails (Pulmonata: Basommatophora). Reprod. Biol. 12, 301–308. [DOI] [PubMed] [Google Scholar]
- Polese G, Bertapelle C, Di Cosmo A, 2015. Role of olfaction in Octopus vulgaris reproduction. Gen. Comp. Endocrinol. 210, 55–62. [DOI] [PubMed] [Google Scholar]
- Ram JL, Gallardo CS, Ram ML, Croll RP, 1998. Reproduction-associated immunoreactive peptides in the nervous systems of prosobranch gastropods. Biol. Bull. 195, 308–318. [DOI] [PubMed] [Google Scholar]
- Rizk MZ, Metwally NS, Hamed MA, Mohamed AM, 2012. Correlation between steroid sex hormones, egg laying capacity and cercarial shedding in Biomphalaria alexandrina snails after treatment with Haplophyllum tuberculatum. Exp. Parasitol. 132, 171–179. [DOI] [PubMed] [Google Scholar]
- Ross LG, Ross B, 2009. Anaesthetic and Sedative Techniques for Aquatic Animals. John Wiley & Sons, 222 pp. [Google Scholar]
- Rothman BS, Mayeri E, Brown RO, Yuan P-M, Shively JE, 1983. Primary structure and neuronal effects of α-bag cell peptide, a second candidate neurotransmitter encoded by a single gene in bag cell neurons of Aplysia. Proc. Natl. Acad. Sci. U. S. A. 80, 5753–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubos EW, Van De Ven AMH, 1987. Morphology of neurosecretory cells in basommatophoran snails homologous with egg-laying and growth hormone-producing cells of Lymnaea stagnalis. Gen. Comp. Endocrinol. 67, 7–23. [DOI] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW, 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleuddin AS, Sevala VM, Sevala VL, Mukai ST, Khan HR, 1992. Involvement of mammalian insulin and insulin-like peptides in shell growth and shell regeneration in molluscs In: Suga S, Watabe N (Eds.), Hard Tissue Mineralization and Demineralization. Springer, pp. 149–169. [Google Scholar]
- Scheller RH, Jackson JF, McAllister LB, Rothman BS, Mayeri E, Axel R, 1983. A single gene encodes multiple neuropeptides mediating a stereotyped behavior. Cell 32, 7–22. [DOI] [PubMed] [Google Scholar]
- Scheller RH, Schaefer M, 1985. Neuropeptide gene expression and behavior in Aplysia In: Selverston A (Ed.), Model Neural Networks and Behavior. Springer, pp. 491–512. [Google Scholar]
- Schindelin J, Arganda-Carreras T, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schot LPC, Boer HH, 1982. Immunocytochemical demonstration of peptidergic cells in the pond snail Lymnaea stagnalis with an antiserum to the molluscan cardioactive tetrapeptide FMRF-amide. Cell Tissue Res. 225, 347–354. [DOI] [PubMed] [Google Scholar]
- Smit AB, Jiménez CR, Dirks RW, Croll RP, Geraerts WPM, 1992. Characterization of a cDNA clone encoding multiple copies of the neuropeptide APGWamide in the mollusk Lymnaea stagnalis. J. Neurosci. 12, 1709–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AB, Vreugdenhil E, Ebberink RHM, Geraerts WPM, Klootwijk J, Joosse J, 1988. Growth- controlling molluscan neurons produce the precursor of an insulin-related peptide. Nature 331, 535. [DOI] [PubMed] [Google Scholar]
- Smith SA, Croll RP 1998. Mollusca In: Adams TS (Ed.), Reproductive Biology of Invertebrates, Volume 8: Progress in Reproductive Endocrinology. John Wiley & Sons, pp. 61–152. [Google Scholar]
- Smith SA, Nason J, Croll RP 1997. Detection of APGWamide-like immunoreactivity in the sea scallop, Placopecten magellanicus. Neuropeptides 31:155–65. [DOI] [PubMed] [Google Scholar]
- Sonetti D, Van Heumen WRA, Roubos EW, 1992. Light- and electron-microscopic immunocytochemistry of a molluscan insulin-related peptide in the central nervous system of Planorbarius corneus. Cell Tissue Res. 267, 473–481. [DOI] [PubMed] [Google Scholar]
- Stuart DK, Strumwasser F, 1980. Neuronal sites of action of a neurosecretory peptide, egg-laying hormone, in Aplysia californica. J. Neurophysiol. 43, 499–519. [DOI] [PubMed] [Google Scholar]
- Vaasjo LO, Quintana AM, Habib MR, Mendez de Jesus PA, Croll RP, Miller MW, 2018. GABA-like immunoreactivity in Biomphalaria: Colocalization with tyrosine hydroxylase-like immunoreactivity in the feeding motor systems of panpulmonate snails. J.Comp. Neurol. 526, 1790–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo D, Habib MR, Delgado N, Vaasjo LO, Croll RP, Miller MW, 2014. Localization of tyrosine hydroxylase-like immunoreactivity in the nervous systems of Biomphalaria glabrata and Biomphalaria alexandrina, intermediate hosts for schistosomiasis. J. Comp. Neurol. 522, 2532–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Golen FA, Li KW, de Lange RP, Jespersen S, Geraerts WPM, 1995. Mutually exclusive neuronal expression of peptides encoded by the FMRFa gene underlies a differential control of copulation in Lymnaea. J. Biol. Chem. 270, 28487–28493. [DOI] [PubMed] [Google Scholar]
- Van Heumen WR, Roubos EW, 1990. Ultrastructural evidence for synthesis, storage and release of insulin-related peptides in the central nervous system of Lymnaea stagnalis. Neuroscience 39, 493–500. [DOI] [PubMed] [Google Scholar]
- Van Heumen WR, Roubos EW, 1991. Immuno-electron microscopy of sorting and release of neuropeptides in Lymnaea stagnalis. Cell Tissue Res. 264, 185–195. [DOI] [PubMed] [Google Scholar]
- Van Minnen J, Reichelt D, Lodder JC, 1979. An ultrastructural study of the neurosecretory canopy cell of the pond snail Lymnaea stagnalis (L.), with the use of the horseradish peroxidase tracer technique. Cell Tissue Res. 204, 453–462. [DOI] [PubMed] [Google Scholar]
- Van Minnen J, Schallig H, 1990. Demonstration of insulin-related substances in the central nervous systems of pulmonates and Aplysia californica. Cell Tissue Res. 260, 381–386. [Google Scholar]
- Van Minnen J, Schallig H, Ramkema MD, 1992. Identification of putative egg-laying hormone containing neuronal systems in gastropod molluscs. Gen. Comp. Endocrinol. 86, 96–102. [DOI] [PubMed] [Google Scholar]
- Van Minnen J, Vd Haar C, Raap AK, Vreugdenhil E, 1988. Localization of ovulation hormone-like neuropeptide in the central nervous system of the snail Lymnaea stagnalis by means of immunocytochemistry and in situ hybridization. Cell Tissue Res. 251, 477–484. [DOI] [PubMed] [Google Scholar]
- Voronezhskaya EE, Elekes K, 2003. Expression of FMRFamide gene encoded peptides by identified neurons in embryos and juveniles of the pulmonate snail Lymnaea stagnalis. Cell Tissue Res. 314, 297–313. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil E, Jackson JF, Bouwmeester T, Smit AB, Van Minnen J, Van Heerikhuizen H, Klootwijk J, Joosse J, 1988. Isolation, characterization, and evolutionary aspects of a cDNA clone encoding multiple neuropeptides involved in the stereotyped egg-laying behavior of the freshwater snail Lymnaea stagnalis. J. Neurosci. 8, 4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhao M, Liang D, Bose U, Kaur S, McManus DP, Cummins SF, 2017. Changes in the neuropeptide content of Biomphalaria ganglia nervous system following Schistosoma infection. Parasit. Vectors 10, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2011. Report of an informal consultation on schistosomiasis control. Geneva, Switzerland: (WHO/HTM/NTD/PCT/2013.3). http://www.who.int/neglected_diseases/resources/9789241505017/en/. Accessed in May 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.